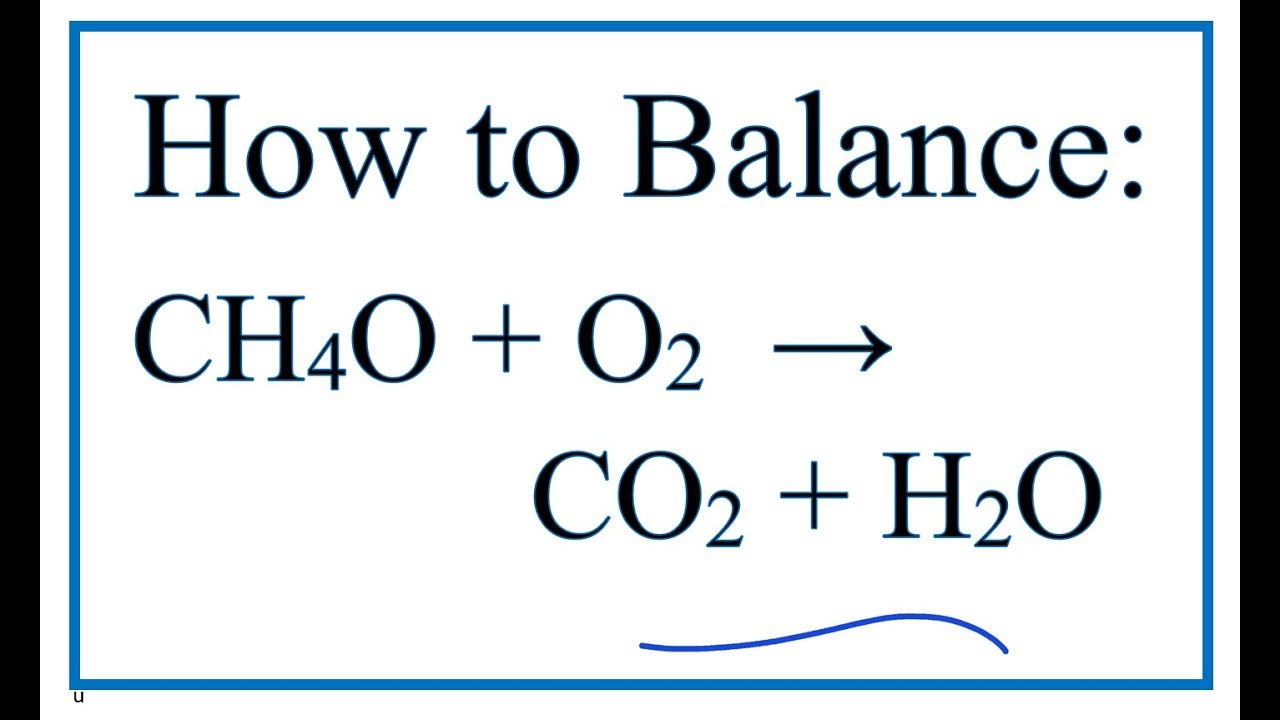
Nice Ch3oh Combustion Formula

There is no H20 so elimate H2O from the equation by using equation 3.
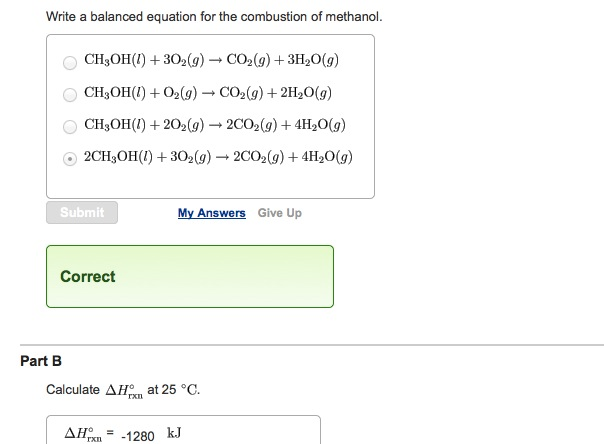
Ch3oh combustion formula. CH3OH l 3O2 g rightarrow CO2 g 3H2O g CH3OH l O2 g rightarrow CO2 g 2H20 g CH3OH l 2O2 g rightarrow 2CO2 g 4H20 g 2CH3OH l 3O2 g rightarrow 2CO2 g 4H20 g Correct Calculate Delta H degree rxn at 25 degree C. NCH3OH X O2 n CO2 2n H2O where n is the number of moles of methanol and X is the number of moles of oxygen required for complete combustion or methanol. When methanol CH3OH is burned in the presence of oxygen gas O2 a large amount of heat energy is released.
Write a balanced equation for the reaction using the AH notation - 2 marks b. 2 CH3OH 3 O2 2 CO2 4 H2O What is the coefficient for O2 when the equation for the combustion of methanol is balanced. 2H2O CO2 --- 32O2 CH3OH Then look at the final equation again.
What is the formation of CH3OH. Equation 3 has 1 H2O 2 and 1 will leave you with 1 left over so the 1 H2O in equation 3 needs to be multiplied by 2 to make it 2 and they cancel. CH3OH and H2CO were formed as major products from the 100 eV electron-irradiated mixed CH4H2O solid at 10 K.
The heat of combustion of methanol CH3OH as described in the equation CH3OH 1½O2g CO2g 2H2O is 715 kJ mol1 and the heats of formation of carbon dioxide gas and water liquid are 3935 kJ mol1 and -2858 kJ mol1 respectively. The combustion reaction of acetylene gas is represented by this equation. It has a role as an amphiprotic solvent a fuel a human metabolite an Escherichia coli metabolite a mouse metabolite and a Mycoplasma genitalium metabolite.
CH3 OH CH3OH and the insertion reaction. The standard enthalpy of formation of liquid methanol CH3OH l ΔHf1 2 ΔHf2 - ΔH3 -3935 kJmol 2 -2858 kJmol - - 72656 kJmol. When methanol CH3OH acts as a base its conjugate acid is ________.
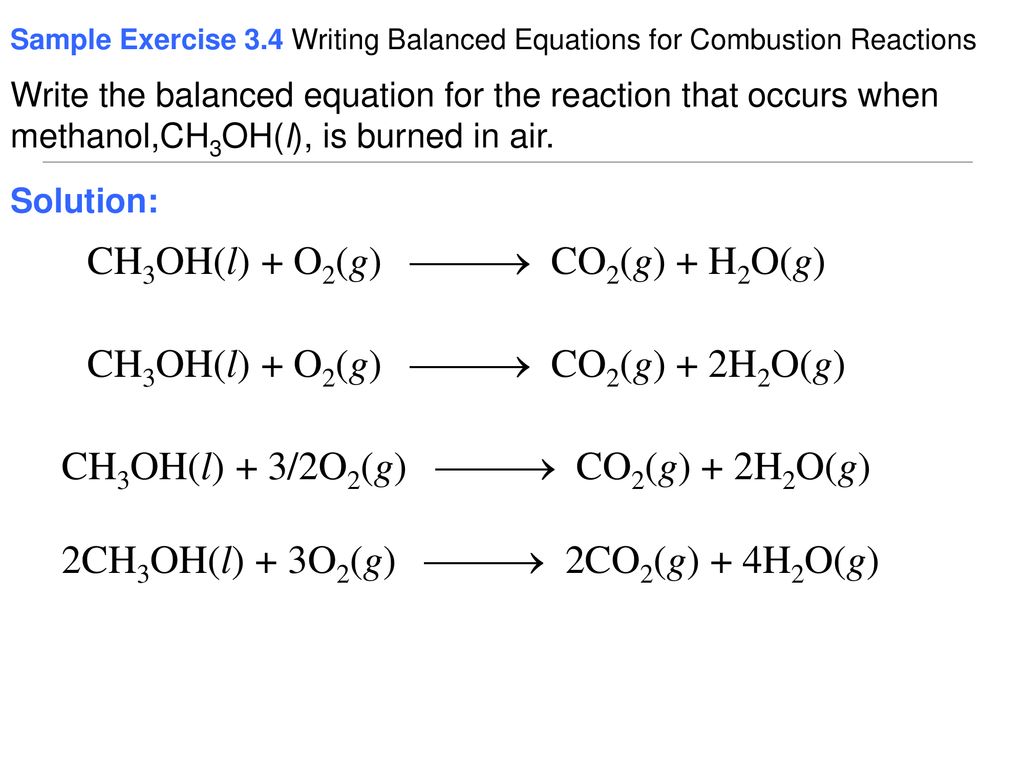
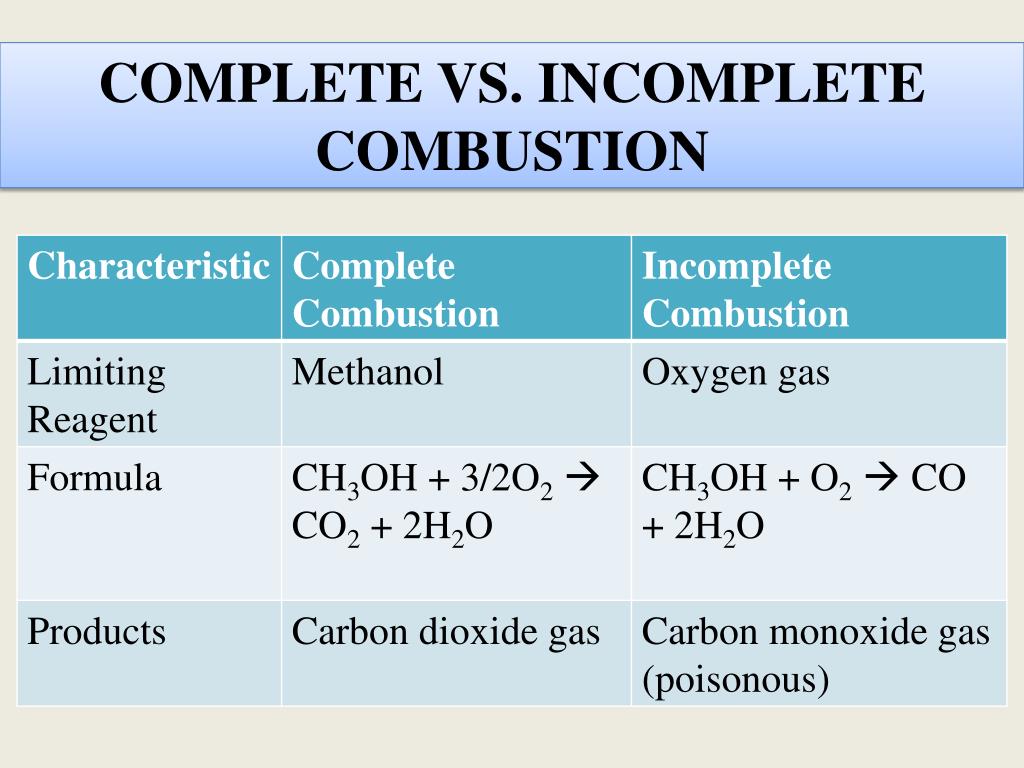
Methanols chemical formula is CH3OH so the basic equation to burn this fuel in oxygen would be. There found to be two pathways for the formation of methanol with about equal importance ie the recombination reaction. Methanol is the primary alcohol that is the simplest aliphatic alcohol comprising a methyl and an alcohol group.