Unbelievable Ammonia And Carbon Dioxide Balanced Equation

Nitrogen dioxide gas with elemental hydrogen to form ammonia gas and water gas.
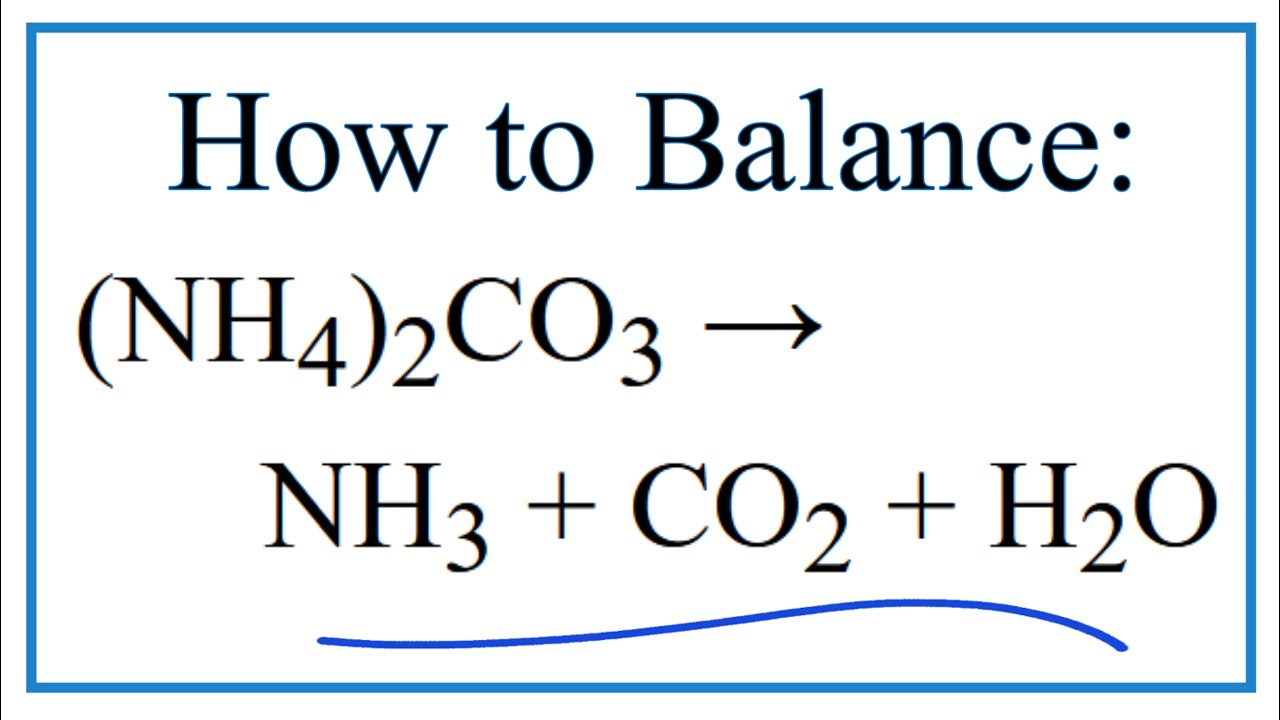
Ammonia and carbon dioxide balanced equation. NH 4 2 CO 3 NH 3 CO 2 H 2 O 18. If a sample of 700 g of carbon oxide was reacted completely with 320 g of oxygen how many moles of carbon dioxide. Part A Enter a balanced equation for thereaction in which gaseous ammonia NH3 and gaseous carbon dioxide react when heated and pressurized to produce aqueous carbamide NH22CO and liquid water.
The products of the. 2HNO3 K2O -- H20 2KNO3 What are similarities between the scheme of work and lesson plan. CH4 2 O2 - CO2 2 H2O What volume of carbon dioxide is produced when 32 L of oxygen are consumed.
Urea is made from ammonia and carbon dioxide. Elemental chlorine with aqueous potassium iodide to form elemental iodine and aqueous potassium chloride. H2O and carbon dioxide CO2.
When combining ammonia gas with carbon dioxide also a gas we get Urea of which the chemical formula is CONH22 and the chemical reaction will be. Magnesium carbonate decomposes into magnesium oxide and carbon dioxide. Write a complete balanced equation for this reaction.
NH2COONH4 ammonium carbamate NH2COONH4. To balance the equation. Causes decomposition of sodium hypochlorite within a few seconds Mellor 2 Supp.
2NH3 CO2 - NH22CO H2O So essentially the same as the formula in Abel Palmers formula. Ammonium carbonate ammonia carbon dioxide water Unbalanced equation. AMMONIUM CARBONATE decomposes when heated to give gaseous ammonia and gaseous carbon dioxide.