Recommendation Define A Displacement Reaction

100 6 ratings Answer.
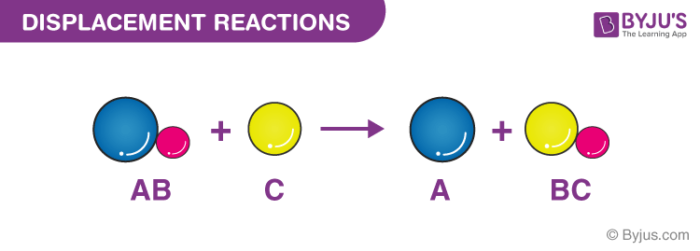
Define a displacement reaction. Definitions a Single Displacement Reaction SDR. Define a single displacement reaction and a double displacement reaction. Itis a type of chemical change wherein an element reacts with a compound and takes the place of another element in that compound.
What are displacement reactions. Write a balanced chemical equation for five of those reactions. Both metals and non-metals take part in displacement reactions.
It is also called a replacement reaction. Displacement reactions Displacement reactions occur when a metal from the electrochemical series is mixed with the ions of a metal lower down in the electrochemical series. There are two types of displacement reactions.
Chemical reaction and equation. Better learning for better results. As replacement of one ion of reactant takes place by another ion of reactant.
What is displacement reaction. Represent view the full answer. A chemical reaction between two compounds in which the first and second parts of one reactant are united respectively with the second and first parts of the other reactant.
Displacement Reaction Definition The type of reaction in which part of one reactant is displaced by another reactant is called displacement reaction. Displacement reaction - a reaction in which an elementary substance displaces and sets free a constituent element from a compound displacement. Displacement reactions involve a metal and a compound of a different metal.