Smart Balance Equation Of Hcl And Naoh

Acetic acid CH_3COOH will react with sodium hydroxide NaOH to produce sodium acetate CH_3COONa and water.
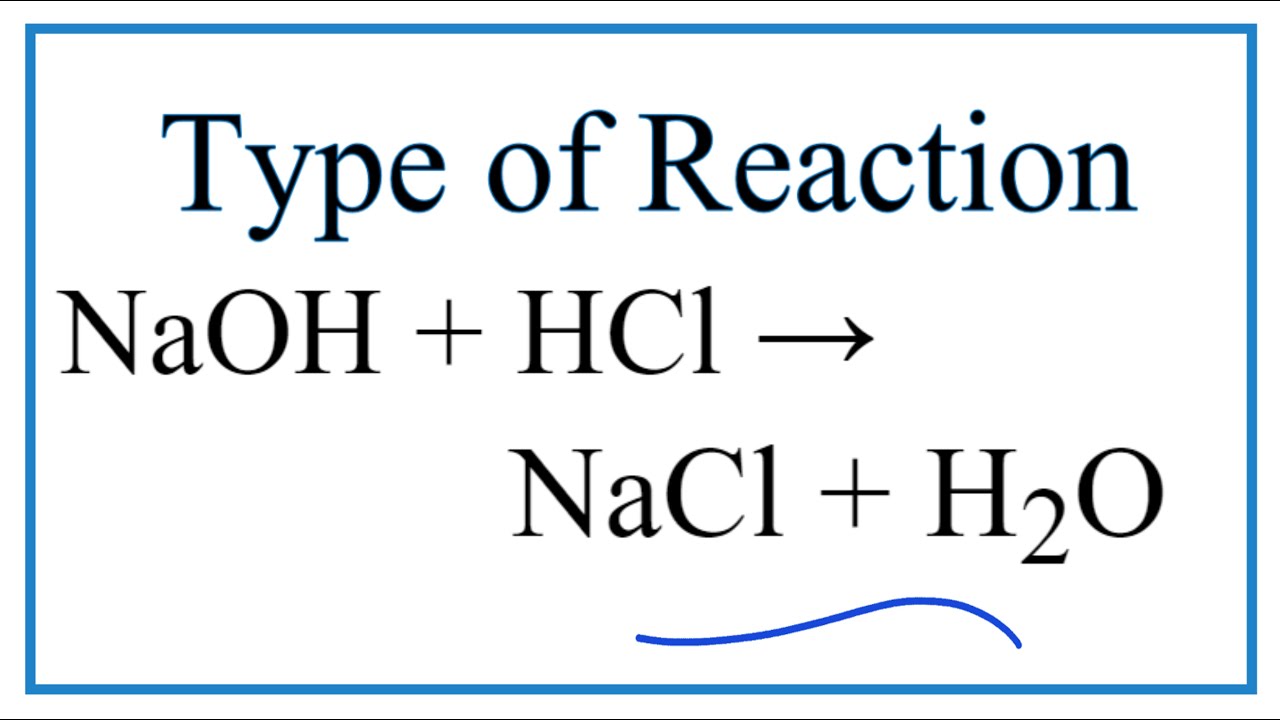
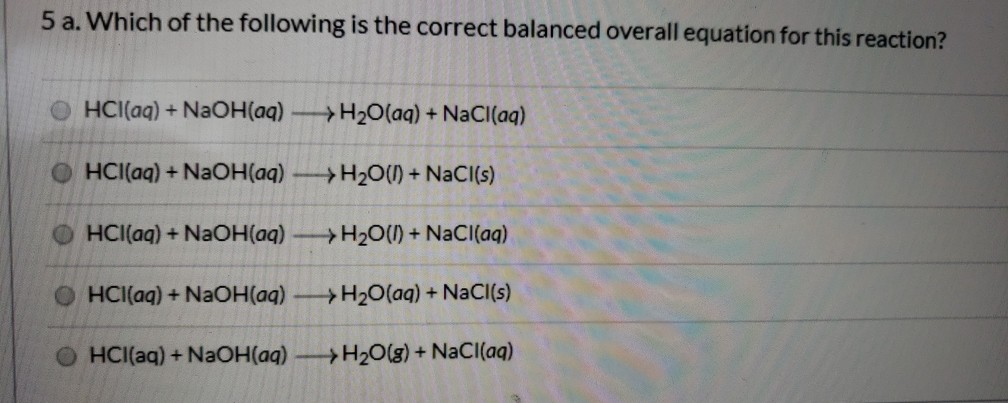
Balance equation of hcl and naoh. HCl aq NaOH aq NaCl aq H2O l heat Since theses are dilute solutions and are mostly water assume that the densities of the solutions and the specific heat capacities of the solutions are approximately 10 gml and 418 JgC respectively. The balanced equation will appear above. Balanced Chemical Equation 2NaOH 2HCl 2NaCl H2O HOH.
We do this by first expressing the chemical formulas of each substance as. What happens when HCl is added to NaCl. This reaction is classified as an exothermic reaction.
For instance in the equation HCl NaOH NaCL H2O the HCl hydrochloric acid a strong acid and NaOH sodium hydroxide a strong base are the reactants. Use uppercase for the first character in the element and lowercase for the second character. What is the balanced equation when hydrochloric acid reacts with sodium hydroxide.
What is the balanced equation for HCl and NaOH. Heres our balanced equation. Examples of complete chemical equations to balance.
Ionic charges are not yet supported and will be ignored. In this equation they react to form the products NaCL sodium chloride or salt and H2O water. The limiting reagent row will be highlighted in pink.
The unbalanced chemical equation that describes this neutralization reaction looks like this CH_ 3COOH_ aq NaOH_ aq - CH_ 3COONa_ aq H_ 2O_ l Now you could check to see if this chemical equation is balanced by. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles.