Breathtaking Luminol Reaction Equation

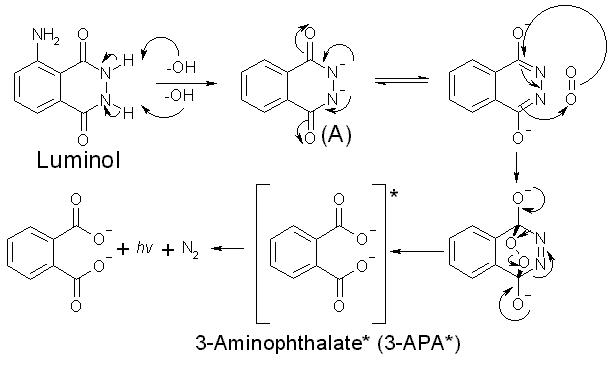
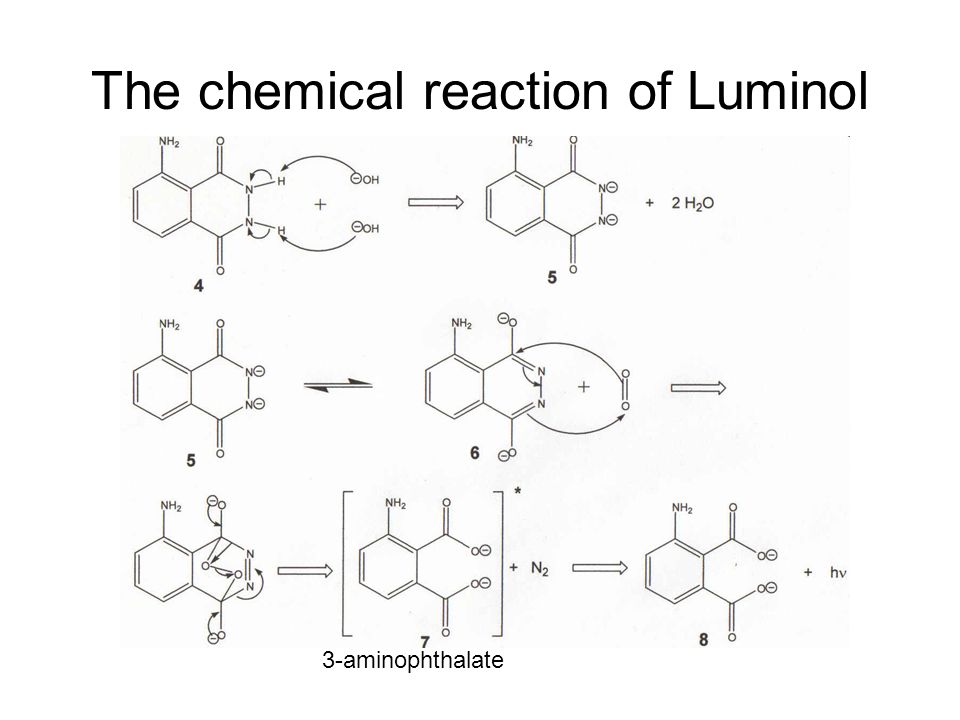
Chemiluminescent reactions of luminol are oxidations occurring under basic conditions as shown in the general equation below.
Luminol reaction equation. The rate constant for the corresponding reaction between oxygen and luminol dianions in aqueous alkali is 10 2 dm 3 mol 1 s 1. Criminalists mix the luminol powder with a liquid containing hydrogen peroxide H2O2 a hydroxide OH- and other chemicals and pour the liquid into a spray bottle. The Chemical Reaction The central chemical in this reaction is luminol C8H7O3N3 a powdery compound made up of nitrogen hydrogen oxygen and carbon.
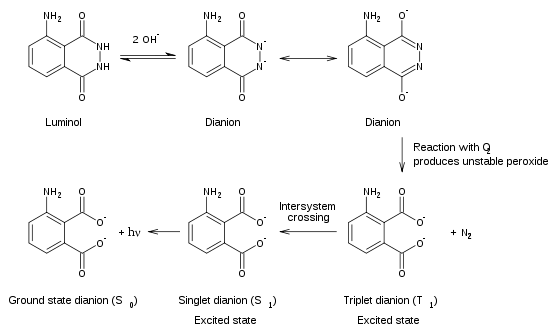
The reaction in this demonstration is a redox reaction in which a photon of light is released from an excited molecule. The luminol reaction could be seen as being made up of two steps. Reactions usually release energy in the form of heat some produce little or no heat and release their energy by the emission of light.
Luminol is a white-to-pale-yellow crystalline solid that is soluble in. Luminol 2NaOH O 2 N 2 Na 2 APA h v White et al have shown that the chemiluminescence of luminol has an emission spectra with two peaks indicating two similar species that emit light. This same reaction occurs in lightening bugs when the chemical luciferase that is in the insects lower abdomen reacts with the oxygen in the air.
Luminol is an interesting molecule because when oxidized it releases energy in the form of light. To this solution is added a solution of a mild oxidizing agent which is 03 hydrogen peroxide in the demonstration below. Luminol solution reacts with blood to produce light.
Luminol C 8 H 7 N 3 O 2 is a chemical that exhibits chemiluminescence with a blue glow when mixed with an appropriate oxidizing agent. The chemical reaction called chemiluminescence is one of the very few reactions where the energy produced through the reaction is given off as light instead of heat. The luminol solution contains both luminol C8H7N3O2 and hydrogen peroxide H2O2.
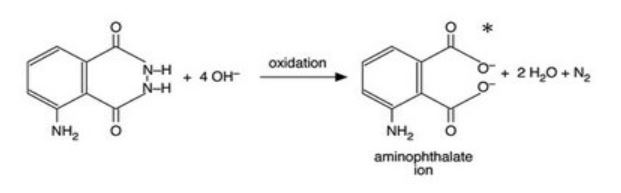
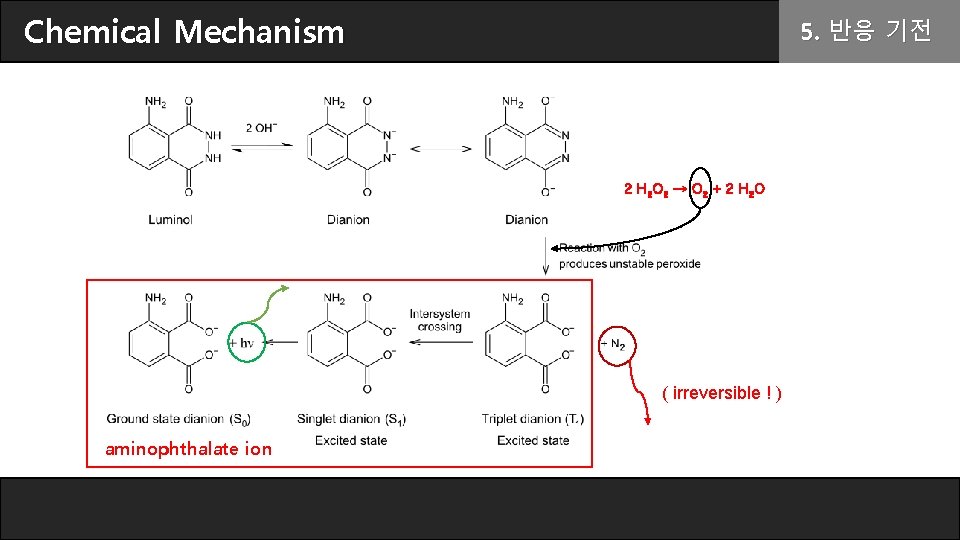
These glowing reactions are generally oxidations and a good example is the oxidation of 5-aminophthalhydrazide or luminol which produces a brilliant blue-green light. NH NH O NH2 O 2 OH 1- O O O NH2 O 2 H2O N 2 In these reactions the blue emitting species has been identified as the aminophthalate ion shown in the above equation. This substance will emit light under certai.