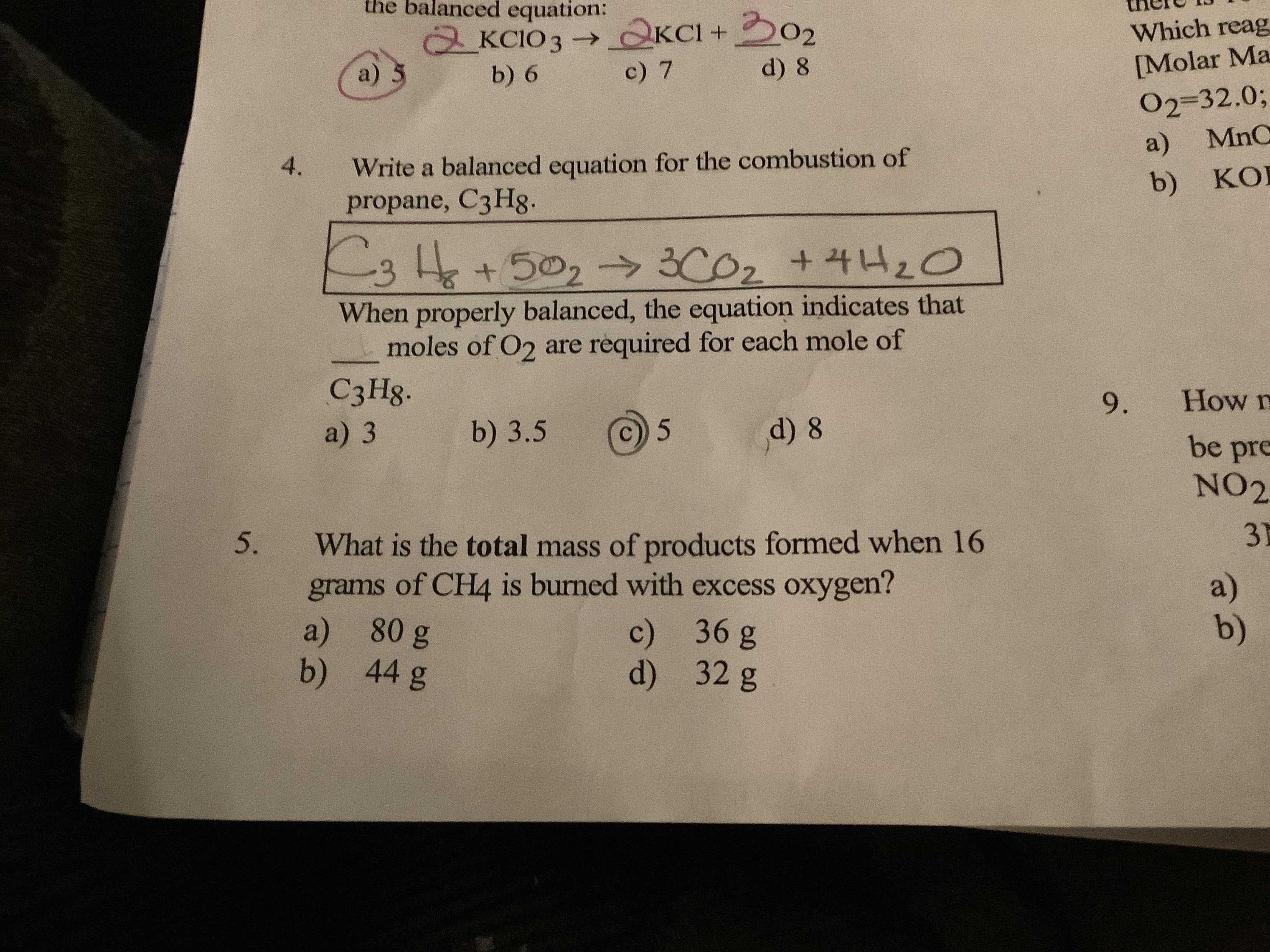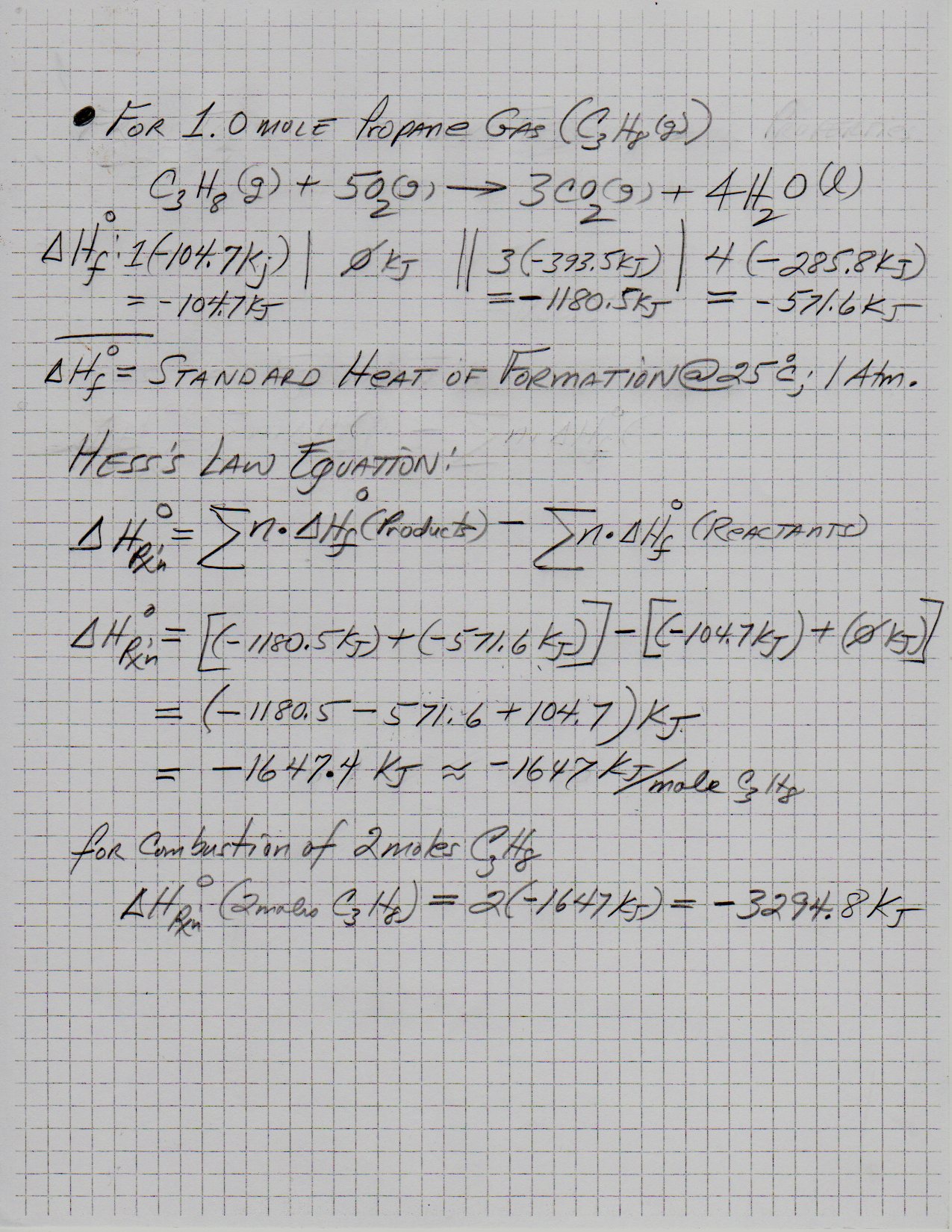Recommendation Balanced Equation For The Combustion Of Propane
Propane camping stoves produce heat by the combustion of gaseous propane C3H8.
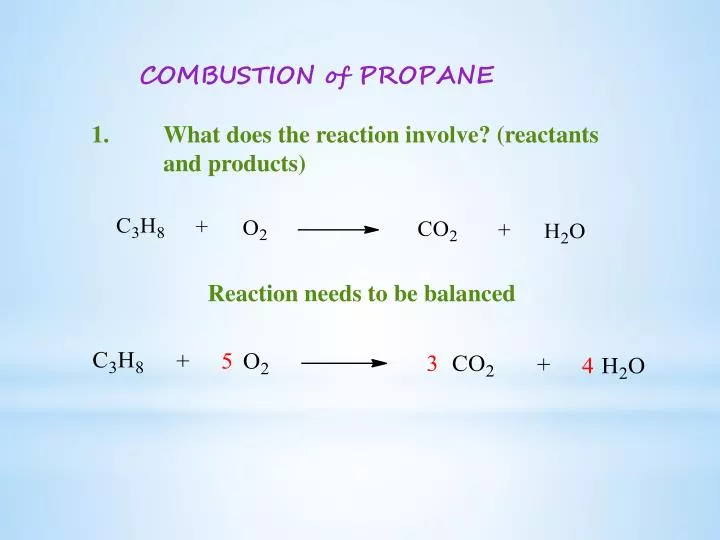
Balanced equation for the combustion of propane. Fuel O2 -CO2 H2O The coefficients of the balanced equation will change depending on the fuel. Butane C4H10 is a component of natural gas that is used as fuel for cigarette lighters. In this video well balance the equation C3H8 O2 CO2 H2O and provide the correct coefficients for each compoundTo balance C3H8 O2 CO2 H2O youll.
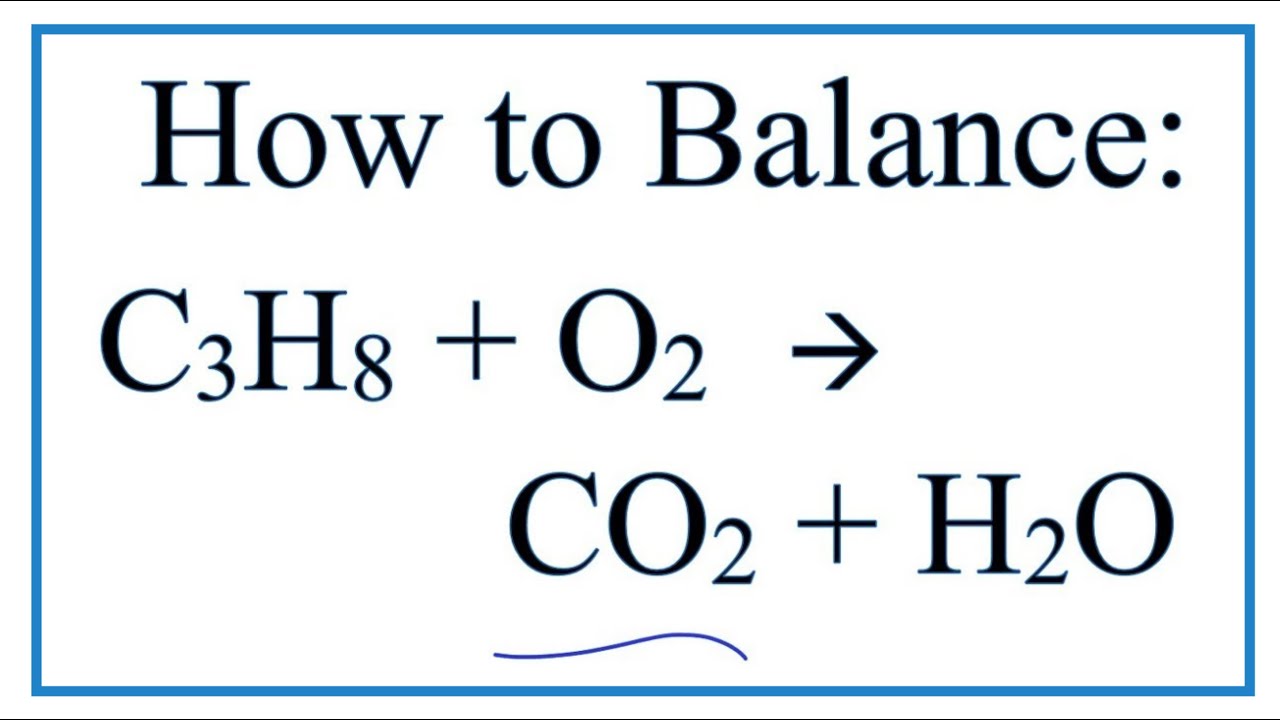
Write a balanced equation for the combustion of gaseous propane C3H8 a minority component of natural gas in which it combines with gaseous oxygen to form gaseous carbon dioxide and gaseous water. Balanced equation for the combustion of propane All combustion reactions fall into the pattern. Balance the skeletal equation for the combustion of propane.
Propane C3H8 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. Write a balanced chemical equation for the combustion of propane c3h8 if you begin with 100l of propane how many liters of oxygen are required for complete combustion write the balanced chemical reaction for the complete combustion of propane c3h8 you can use shorthand notation a make sure to include all reactants and all products b. But it also produces carbon monoxide.
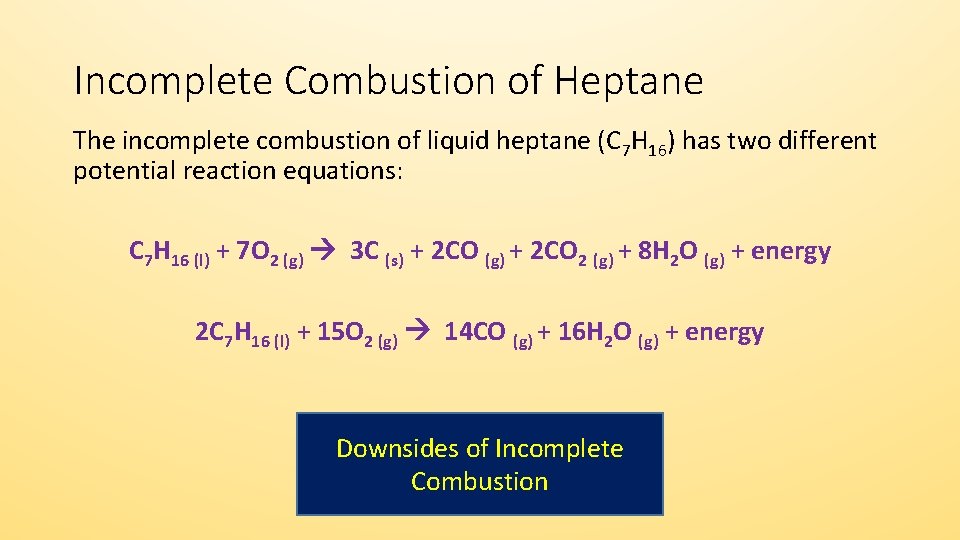
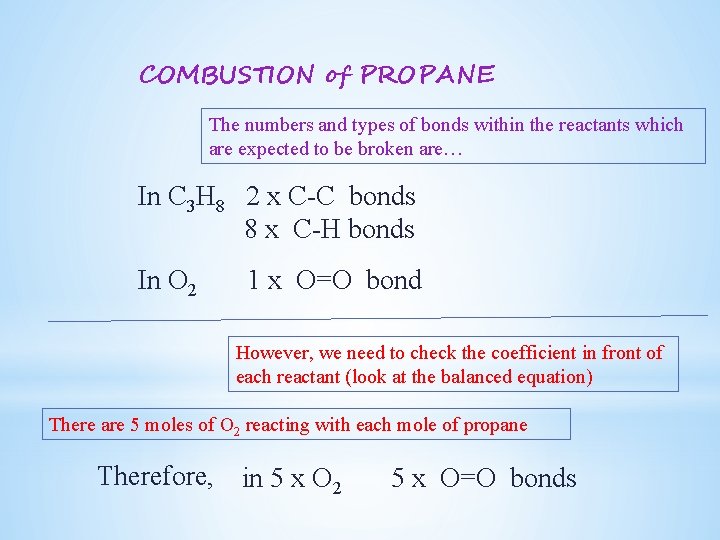
The incomplete combustion of propane C 3 H 8 produces two possible balanced equations. What is the balanced equation for c3h8 o2 co2 h2o. Propane oxygen carbon dioxide water C3H8 5O2 3CO2 4H2O.
The complete combustion of propane produces carbon dioxide gas and water vapor. C 3 H 8 3 O 2 2CO C 4H 2 O C 3 H 8 25 O 2 CO 2C 4H 2 O b Incomplete Combustion Equation -. Represent this reaction with balanced chemical equation.
There will be 10 oxygens on the products side and 2 on the reactants so to balance these out we multiply the 02 on the reactants side by 5. 2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat. For example here is one possible equation for the incomplete combustion of propane.