Recommendation Word Equation For Catalytic Decomposition Of Hydrogen Peroxide

Or sodium iodide as a catalyst to speed up the decomposition of hydrogen peroxide.
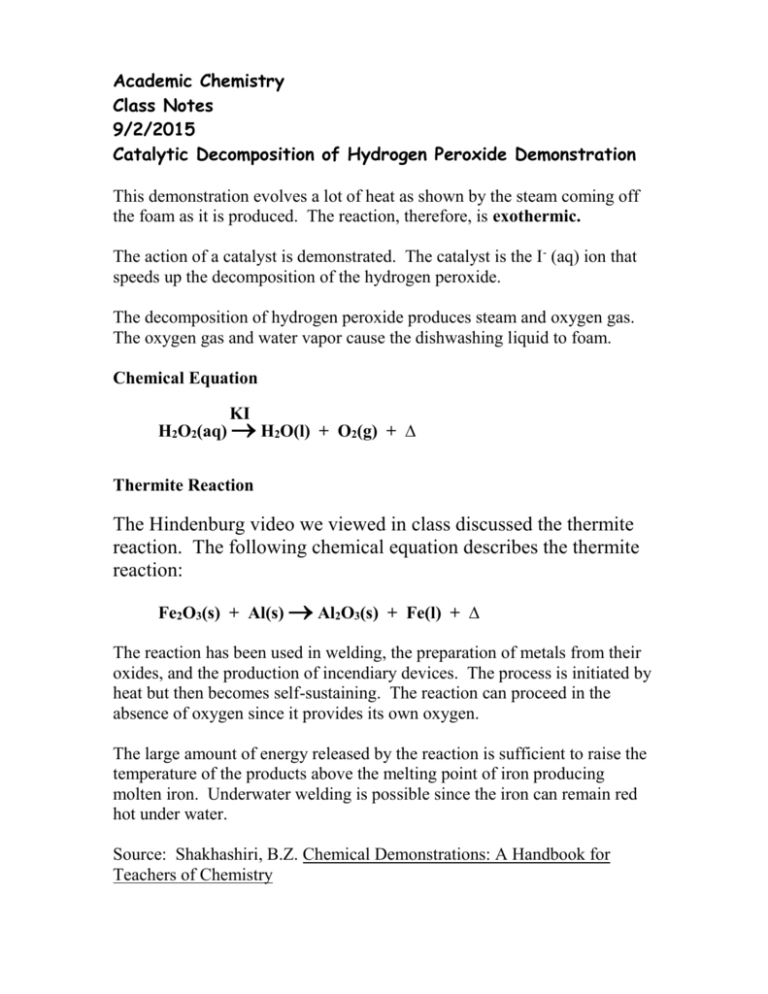
Word equation for catalytic decomposition of hydrogen peroxide. H 2 O 2 aq I- aq H 2 O l OI - aq H 2 O 2 aq OI- aq H 2 O l O 2 g I - aq. The answer to this is number 2. Hydrogen peroxide undergoes disproportionation.
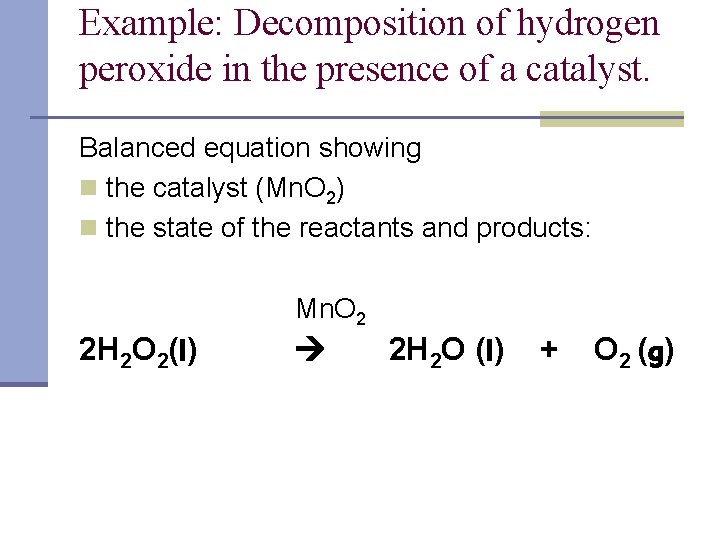
Both oxidation and reduction occur at the same time. 2 H 2 O 2 aq. The skeleton equation is H2O2 MnO2 MnH2 O2 I need help with identifying whether this reaction is a single displacement or a decomposition MnO2 is a catalyst and why.
2H202 is actually the scientific formula for Hydrogen Peroxide. 2H 2 O 2 aq -- 2H 2 O l 12 O 2 g The catalytic decomposition of hydrogen peroxide occurs when administered to wounds. The catalytic decomposition of hydrogen peroxide can be essentially explained by two different mechanisms based on the mutual redox transitions FeIIIFeV KREMER-STEIN mechanism and FeIIIFeII HABER-WEISS mechanism respectively.
Even water exerts a distinct Catalytic Decomposition of hydrogen peroxide effect. The decomposition takes place according to the reaction below. Hydrogen peroxide oxygen water.
2 H 2 O 2aq O 2 g 2 H 2 O l The rate of this reaction can be followed by. Comparison and description of the most commonly used catalysts were presented in this review. Catalytic Decomposition of H 2O2 Elephants Toothpaste Description.
The catalyzed reactions are shown below. It is important that you know how to balance chemical equations to help you understand further why matter cannot be created and destroyed. The resulting products are water and oxygen gas.