Divine Neutralization General Equation

When a strong acid and a strong base fully neutralize the pH is neutral.
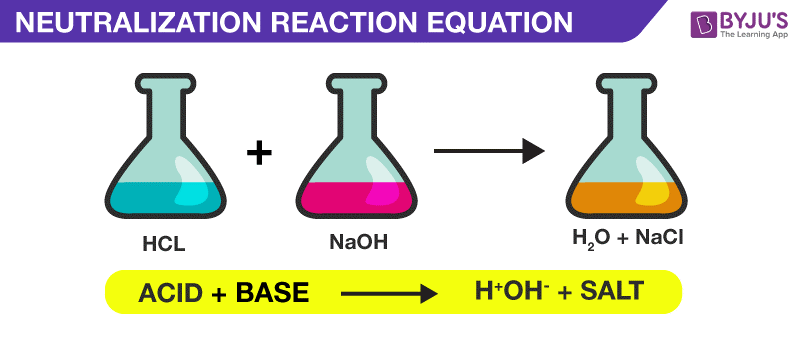
Neutralization general equation. The reaction between an acid and a base that forms water and salt is neutralisation. We know about acids and bases and we know about acid-base reactions so what is a neutralization reactionWatch the whole General Chemistry playlist. NaOH HCl H 2 O and NaCl Now lets break this reaction down into two parts to see how each product forms.
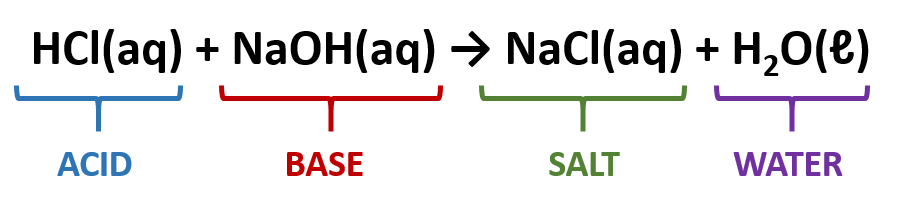
ACID aq BASE aq H 2 O l SALT aq or s The DRIVING FORCE for a general acid-base reaction is the formation of water. It explains how to balance the chemical equation. So when ammonia is one of the reactants we do not include water as a product.
Neutralization Reactions This is the general format for a neutralization reaction. There is no excess NaOH. In acid-alkali neutralisation reactions hydrogen ions from the acid react with hydroxide ions from the alkali.
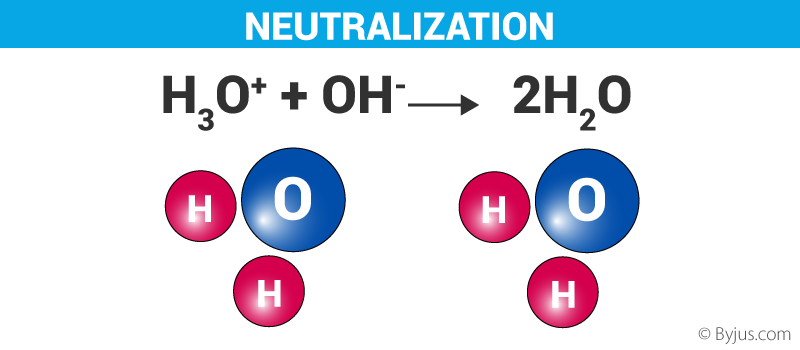
Neutralization Reactions and Net Ionic Equations for Neutralization Reactions. This chemistry video tutorial explains how to predict the products of acid base neutralization reactions. Haq OH-aq H2O l Pure water is neutral its pH is 7.
Lets see how a neutralization reaction produces both water and a salt using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. H OH- H 2 O The neutralisation reaction or neutralization reaction takes place when an. Net neutralization reactions of ionic equations include solid bases solid salts water and solid acids.
This problem has been solved. Metal oxides act as bases. The overall equation for this reaction is.