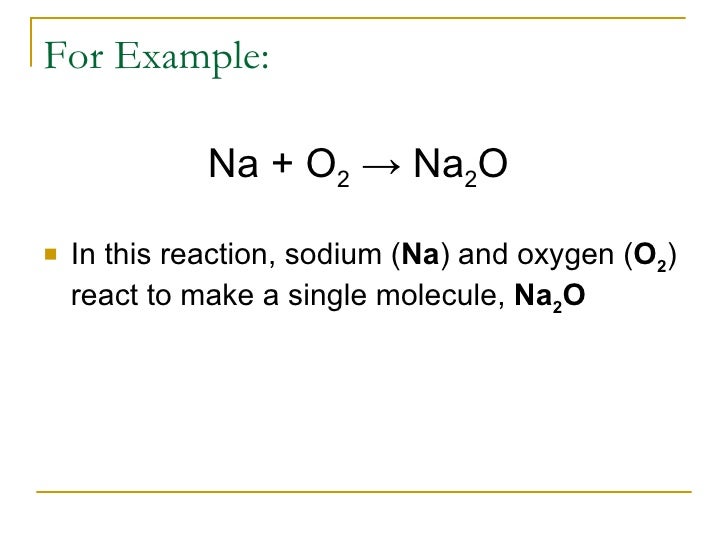Stunning Sodium And Oxygen Balanced Equation

Sodium metal reacts with oxygen gas to produce the compound sodium oxide.
Sodium and oxygen balanced equation. B sodium peroxide water gives sodium hydroxide oxygen gas. Of atoms in ReactantsNo. Below are more examples of balanced chemical equations showing state symbols.
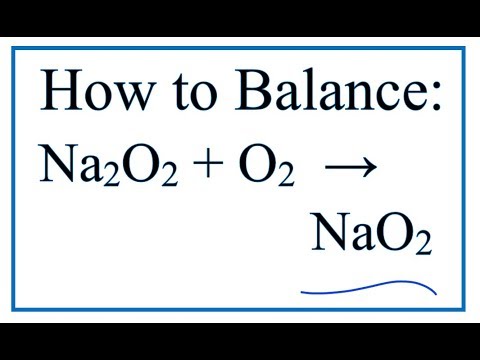
4 Na O 2-- 2 Na2O. Or if any of the following reactant substances NaNO3 sodium nitrate disappearing. For this reaction we have a decomposition reaction.
A page showing Balanced Chemical Equations for The reactions between Sodium and Oxygen Aluminium and Chlorine Aluminium and Oxygen Calcium and Chlorine Magnesium and Bromine. Will sodium reacts with oxygen. The balanced chemical equation for this reaction is given by.
Sodium is a very reactive metal it tends to react with oxygen to form sodium oxide but this is an unstable compound and soon reacts with hydrogen to form sodium hydroxide. In this video well balance the equation Na O2 Na2O and provide the correct coefficients for each compoundTo balance Na O2 Na2O youll need to be su. Sodium reacts with oxygen to form sodium oxide and has the following balanced chemical equation.
An alternate method for the preparation of sodium oxide is by reacting sodium nitrite with sodium. If 598g of sodium react with water to form26g of hydrogen and 1040g of sodium hydroxide what mass of water was consumed in the. However we can assume that different numbers of reactant molecules or product molecules may be involved.
Eq4Na s O_2 g to 2Na_2O s eq From the balanced reaction 4. 6Na 2NaNO 2 N 2 4Na 2 O. 380 - 500C Catalyst.