Matchless Reaction Of Ammonia And Hydrogen Chloride

Answered Reaction of ammonia and hydrogen chloride 1.
Reaction of ammonia and hydrogen chloride. By its chemical nature the nitrogen in ammonia prefers to be attached to four hydrogens rather than the mere three it has so it steals the hydrogen from hydrogen chloride. Two gases forming a solid. In water the reaction between ammonia NH 3 and hydrogen chloride HCl is a textbook example of acid-base chemistry.
Reaction of ammonia and hydrogen chloride Get the answers you need now. Declan Fleming revisits the classic diffusion demo of the reaction of ammonia with hydrogen chloride providing some great tips for doing it even better. This reaction is spontaneous at all temperatures.
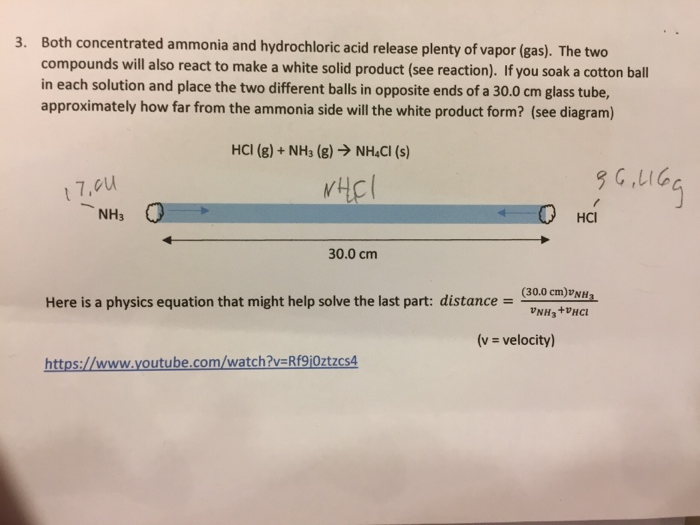
NH3g HCl g NH4Cls. Reaction of ammonia and hydrogen chloride. Chemistry 20052020 1457 xojade.
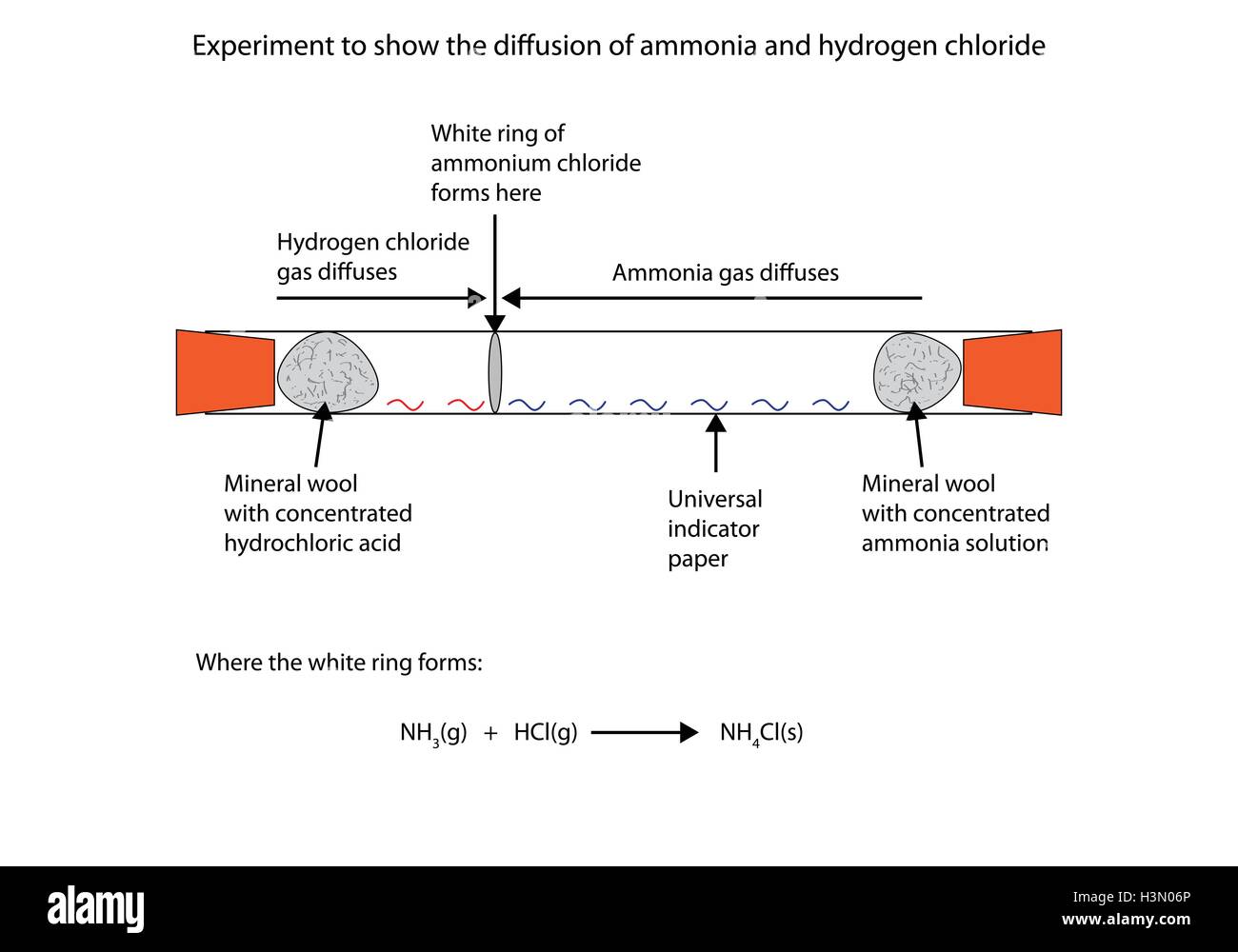
NH3 HCl NH4Cl The white fumes are ammonium chloride gas which on cooling should give ammonium chloride crystals. As ammonia is a base and hydrogen chloride is acidic they react together via a proton transfer reaction resulting in a salt formed ie. Proton transfer plays a key role in a wide range of chemical and biological reactions.
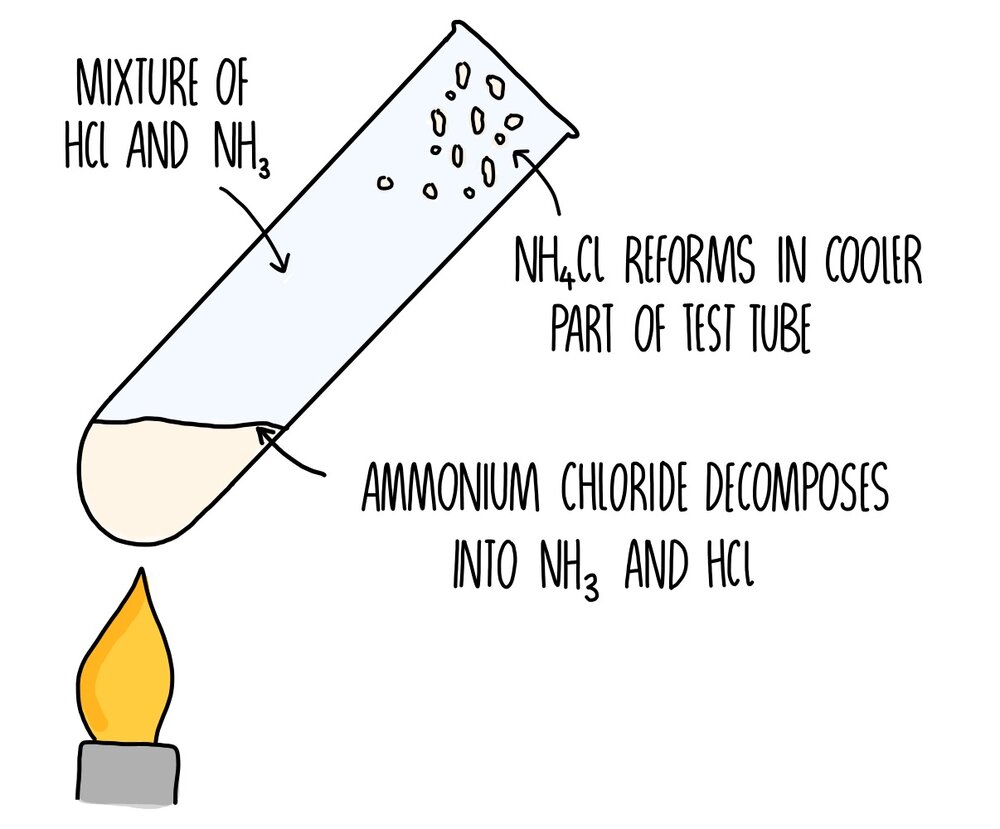
2 stoppered Erlenmeyer flasks with ammonia and hydrogen chloride gases are opened and the product of the reaction ammonium chloride white smoke will appea. Ammonium chloride ammonia hydrogen chloride NH4Cl s NH3g HCl g The equation shows that ammonium chloride a white solid can break down to form ammonia and hydrogen chloride. The reaction is thus.
In water the reaction between ammonia NH3 and hydrogen chloride HCl is a textbook example of acid-base chemistry most of us learn in high school. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is. Kardash kardash 05202020 Chemistry High School 5 pts.