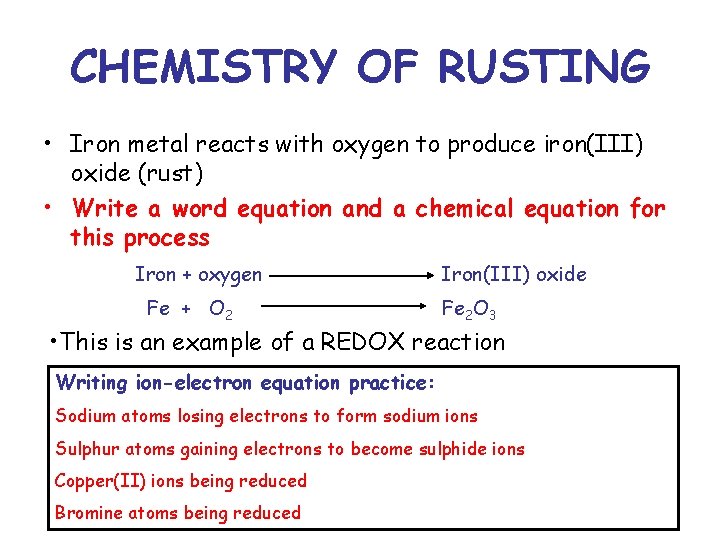
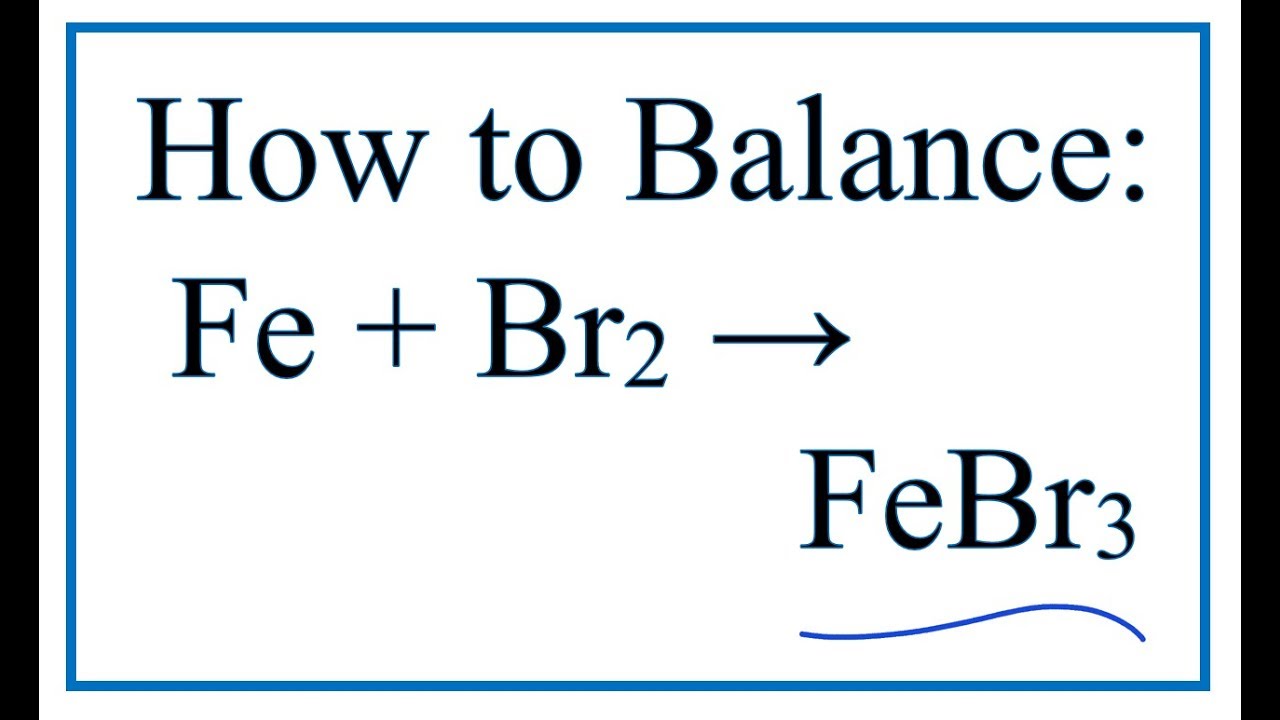Glory Iron And Bromine Reaction Equation
Several hydrates of FeBr 2 are also known all being pale colored solids.
Iron and bromine reaction equation. Give a reason for your choice. Benzene reacts with chlorine or bromine in the presence of a catalyst replacing one of the hydrogen atoms on the ring by a chlorine or bromine atom. I In an experiment 560g of iron reacted exactly with 240g of bromine Br2.
IronII bromide is an inorganic compound with the chemical formula FeBr 2The anhydrous compound is a yellow or brownish-colored paramagnetic solid. There are two possible equations for the redox reaction between iron and bromine. An analogous addition reaction between benzene and bromine.
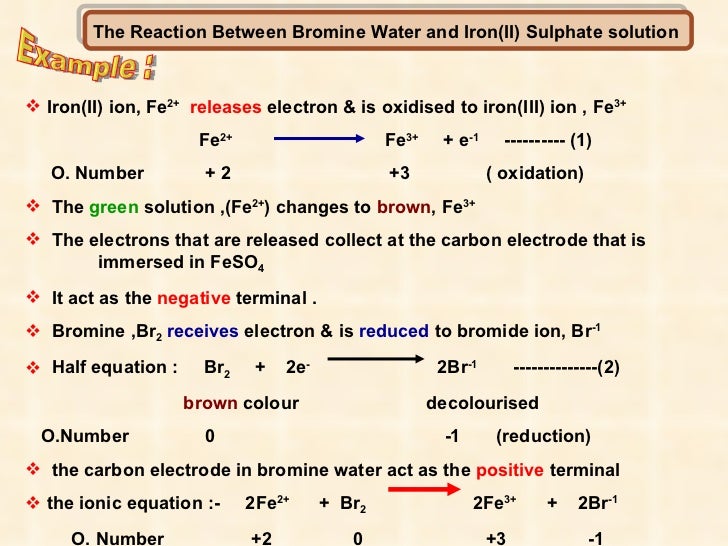
The pale green solution containing the iron II ions turns into a yellow or orange solution containing iron III ions. Fe 560 Br 800 Determine using this information the balanced equation for the reaction between iron and bromine. The reaction with chlorine.
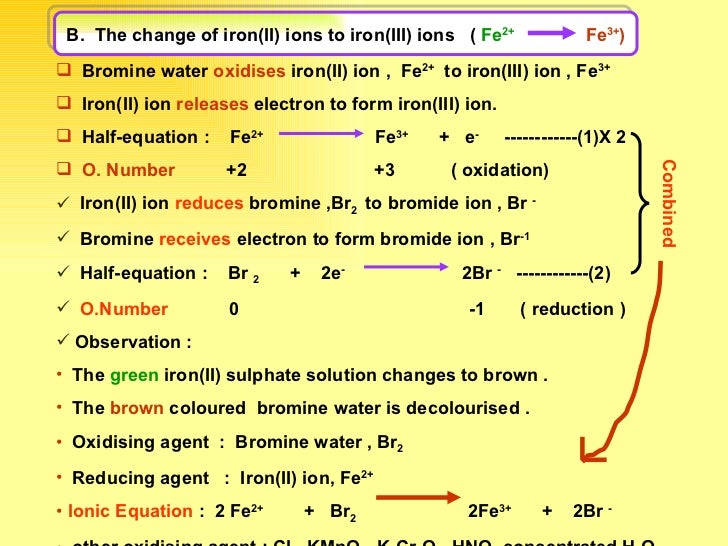
As bromine addition to ethene propene and bromine reaction occurs in same way. Propene and bromine reaction mechanism. Iron II ion Fe 2 undergoes oxidation by releasing an electron to form iron III ion Fe 3.
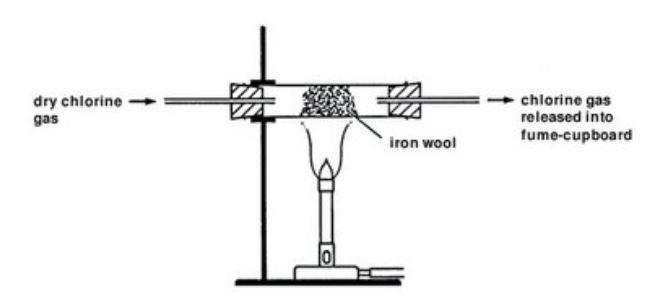
Ch17 Reactions of Aromatic Compounds landscapedocx Page5 Comparison with Alkenes Alkenes react spontaneously with bromine to give addition products. For the bromine equation Br is substituted for Cl. The general equation for the reactions involved is.
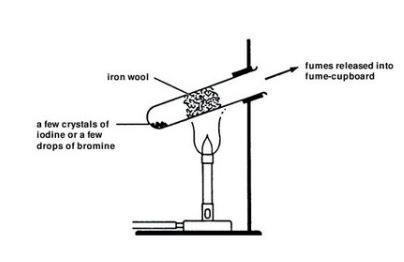
2Fe s 3X 2 g 2FeX 3 s X Cl Br and I. Iron is a transi. Iodine isnt a strong enough oxidising agent to oxidise ironII ions so there is no reaction.