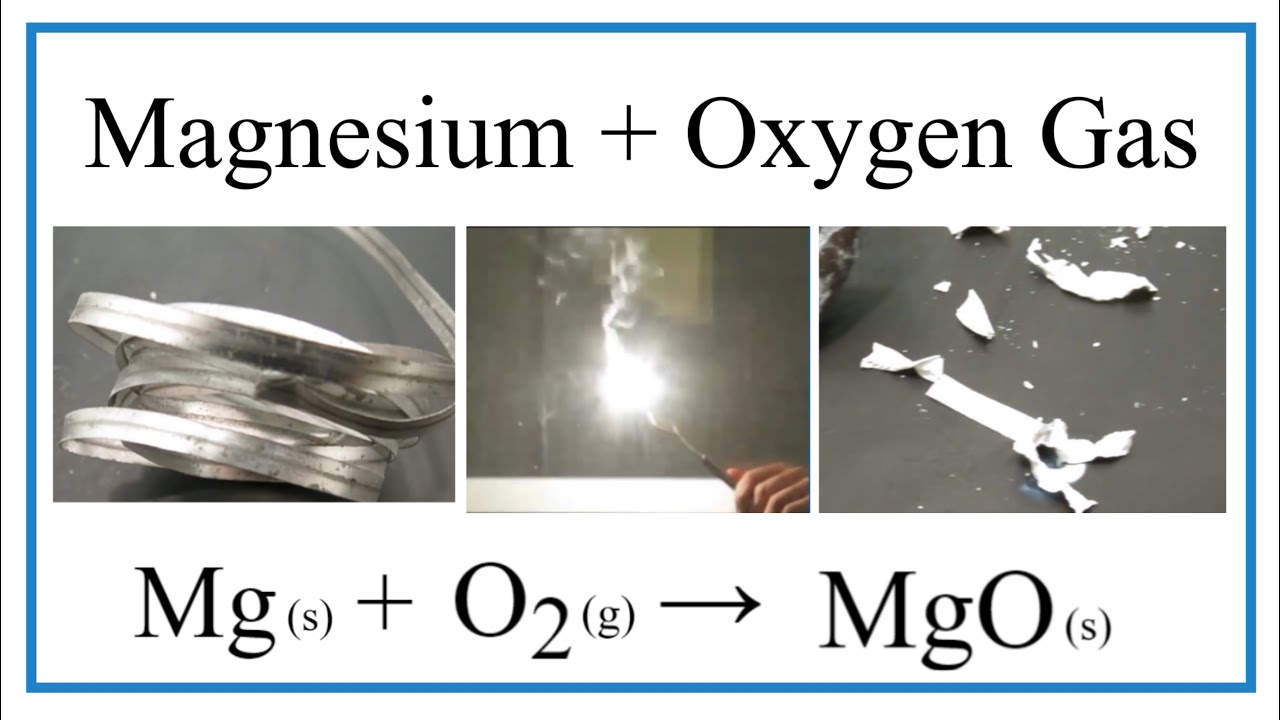
Brilliant Magnesium Metal With Oxygen Gas
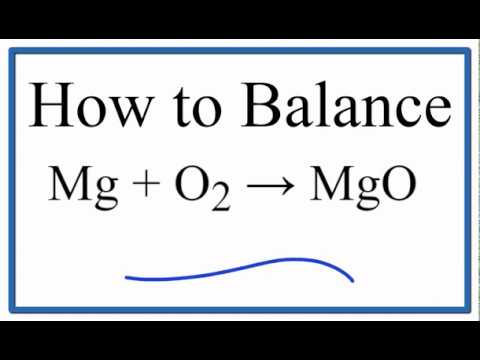
Question 5 1 pts Write the balanced equation for the reaction of magnesium metal with oxygen gas to form magnesium oxide.
Magnesium metal with oxygen gas. If we double the amount of Magnesium it will also double the amount of MgO we will obtain. This means that magnesium has 2 electrons in its outer shell making it highly reactive to oxygen. Write down the name and chemical formula of the product of the reaction.
Here is a short video showing the reaction of magnesium and oxygen. After it burns it forms a white powder of the. What is the coefficient in front of the magnesium oxide.
As per the equation 2 moles of Magnesium produces two moles of MgO. 2 Mg O2 -- 2MgO. We review their content and use your feedback to keep the quality high.
Oxygen and magnesium combine in a chemical reaction to form this compound. Magnesium is in Group 2 of the periodic table. The picture is not the same for all reactions of metals with oxygen.
B How many grams of oxygen are needed to react with 250 g of Mg. How many grams of MgO will result. Magnesium has a very energetic combustion reaction with oxygen where two atoms of magnesium bond with one molecule of oxygen gas to form two molecules of magnesium oxide.
2M gs O2g 2M gO Oxygen is a diatomic molecule and is almost always reacting with elements as a gas O2. Simple composition or formation Reaction between magnesium metal and oxygen gas finishing with balanced equation. This is an exothermic reaction.