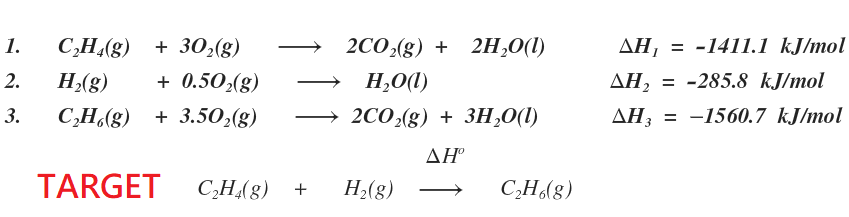Favorite Hess Law Explained
In other words the enthalpy change of a chemical reaction the heat of reaction at constant pressure does not depend on the pathway between the initial and final states.
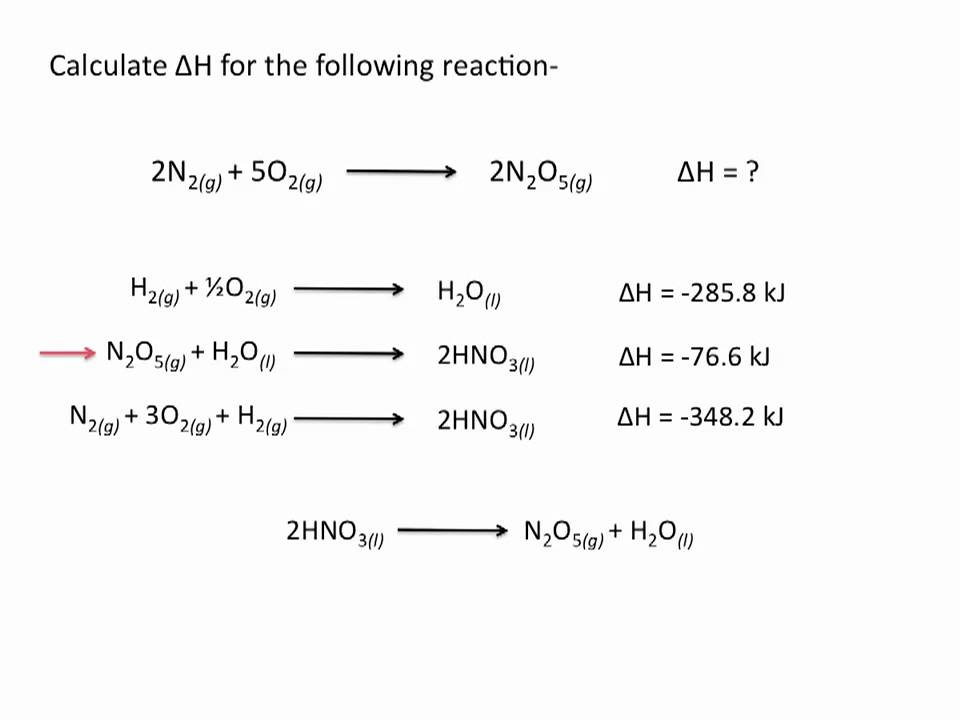
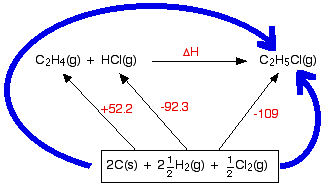
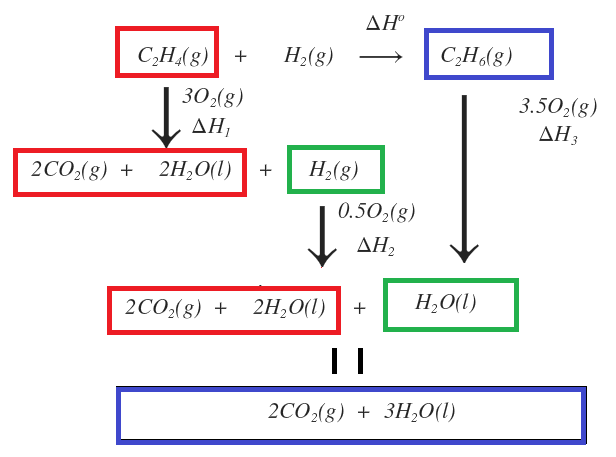
Hess law explained. Hess corporation and its affiliates disclaim all warranties representations and endorsements expressed or implied with regard to information accessed from or via this site including but not limited to all express and implied warranties including the warranty of title merchantability fitness for a particular purpose non-infringement and. The 1st reaction of the three reactions in the Hesss Law problem breaks C2H6 apart to its elements Cs and H2g. Hess law also known as Hesss law of constant heat summation states at constant temperature heat energy changes enthalpy ΔHrec accompanying a chemical reaction will remain constant irrespective of the way the reactants react to form product.
This is how we can make sure a reaction wont explode in ou. What is Hess Law. Hesss Law of Constant Heat Summation or just Hesss Law states that regardless of the multiple stages or steps of a reaction the total enthalpy change for the reaction is the sum of all changes.
In this video I will introduce Hesss Law in a simple and easy to understand way that will help you understand what Hesss Law is and how to perform calculat. The Avvo Rating explained. Hesss law states that the standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction can be divided while each occurs at.
Comments of Amerada Hess Corporation Hess in the above entitled case. It states that the total change in enthalpy ie. Created by Sal KhanWatch the next lesson.
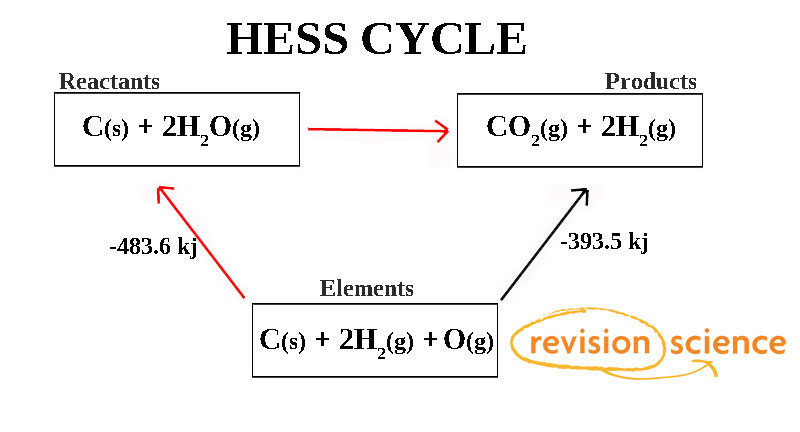
Hess Po Box 3368 Evergreen CO 80437-3368. This law is a manifestation that enthalpy is a state function. Hesss law states that the energy change in an overall chemical reaction is equal to the sum of the energy changes in the individual reactions comprising it.
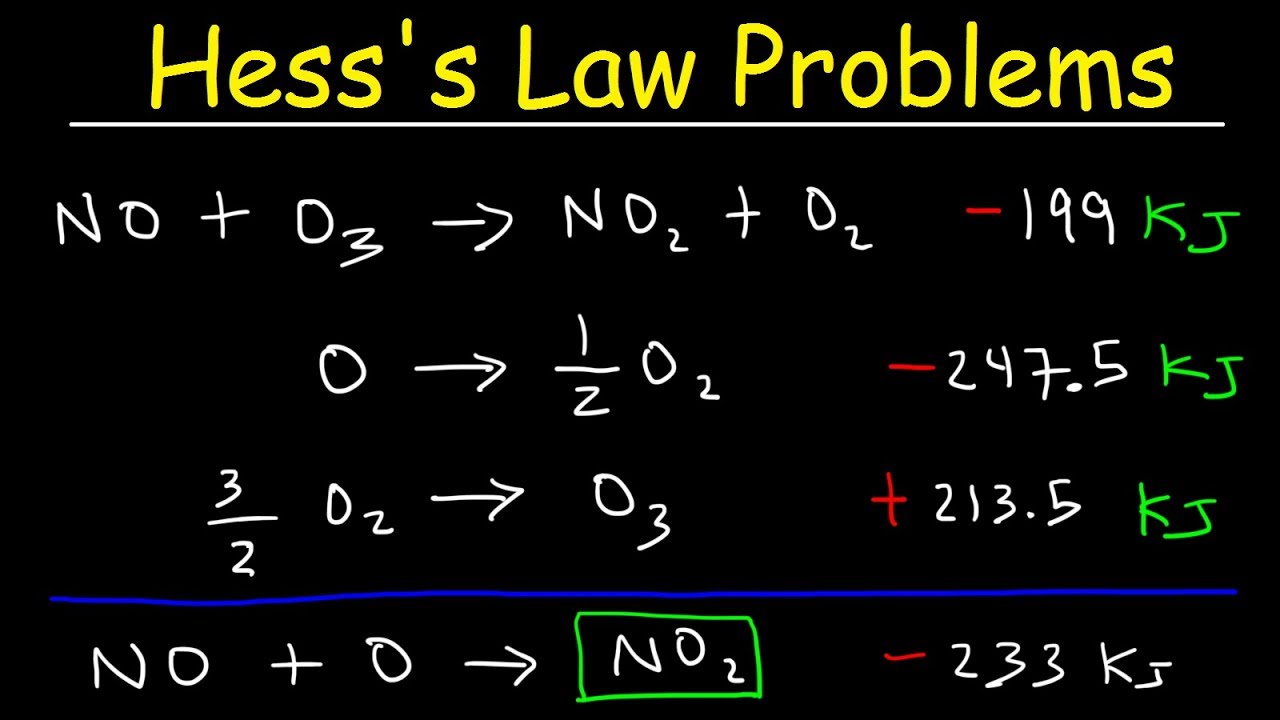
The reaction was flipped and ΔH changed sign Hesss Law has no reaction for the breaking down and forming of O2g since it is already in its elemental state. Endorsements from fellow lawyers are. So you can calculate the enthalpy as the sum of several small steps.