Divine Define Balanced Equation

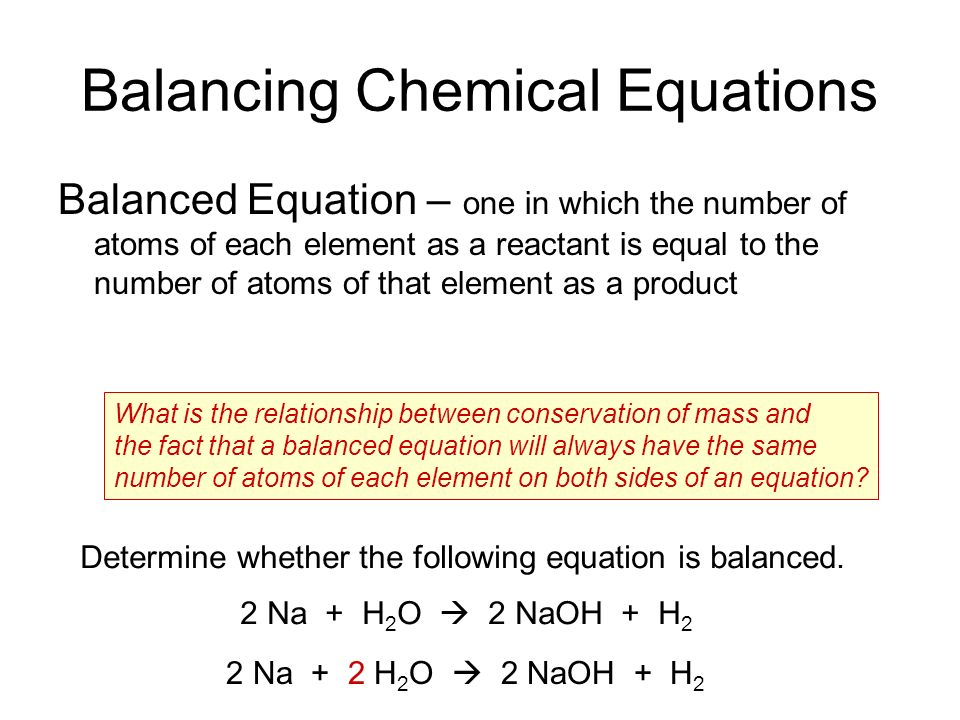
Answer A balanced chemical reaction is an equation that has equal numbers of each type of atom on both sides of the arrow.
Define balanced equation. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. From the Cambridge English Corpus A properly balanced equation would take these factors into account. A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side.
Answers 2 Match the descriptions in the first columns with the terms in the second column. Answers 1 Which of the following is NOT a chemical reaction. A chemical equation that has an equal amount of each element on the left and right sides 2.
B i P4 s 10Cl2 g 4PCl5 S. Use uppercase for the first character in the element and lowercase for the second character. Baking a cake 3.
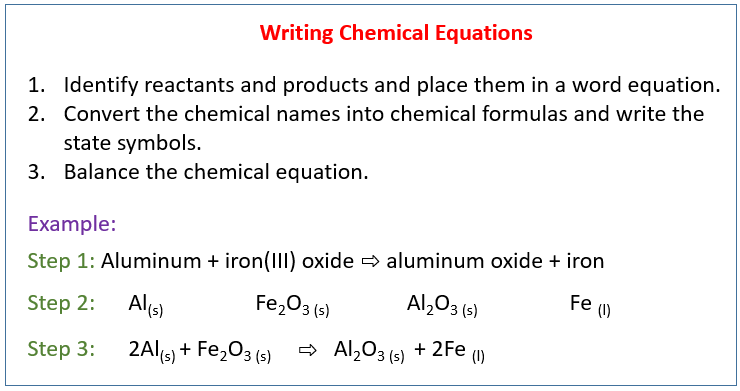
A Define a balanced chemical equation. Write the equation in terms of all of the ions in the solution. According to law of conservation of mass matter can neither be created nor be destroyed in a chemical reaction.
Five years before Boltzmann James Clerk Maxwell used the principle of detailed balance for gas kinetics with the reference to the principle of. This is the British English definition of balanced equation. In other words break all of the strong electrolytes into the ions they form in aqueous solution.
One such method uses oxidation states and a second is referred to as the half-reaction method. Ii Burning of natural gas. First column Forms when electrons are shared unequally between two.