Looking Good Coefficients In A Chemical Formula
H2 O2 H2O Chemical Equation Balancer.
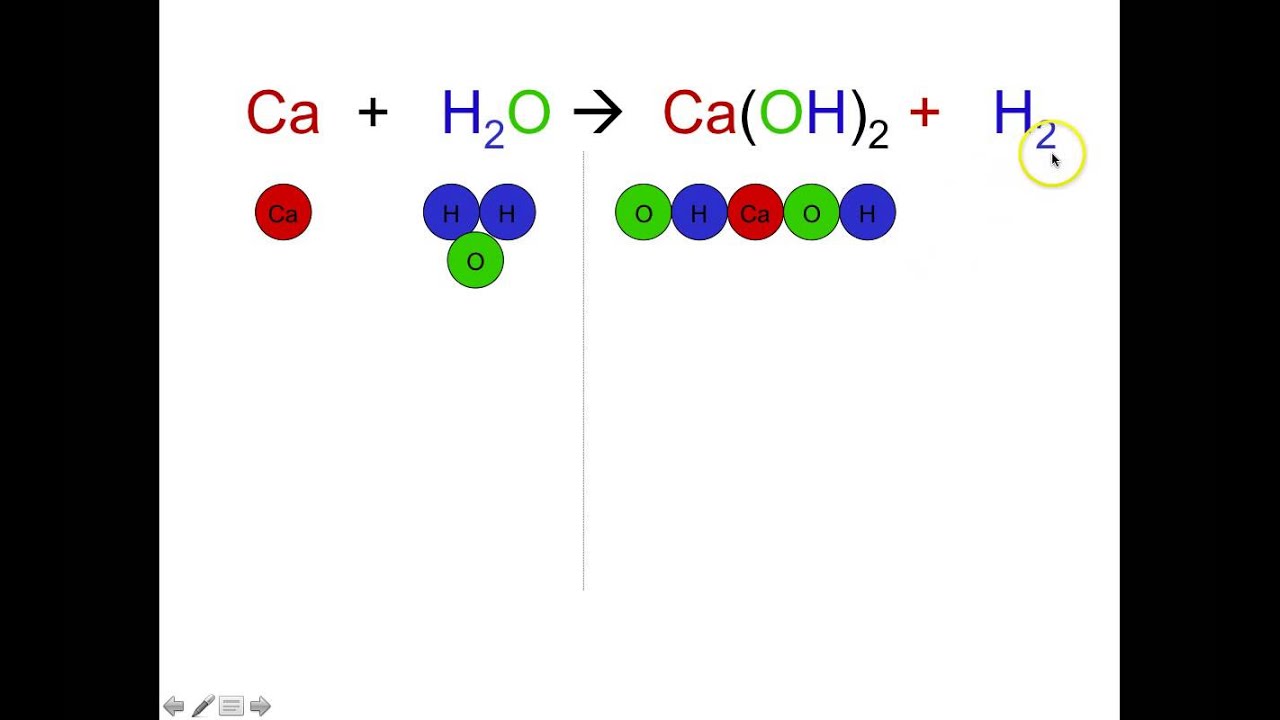
Coefficients in a chemical formula. They are used to multiply all the atoms in a compound In the following formula which is the coefficient. Understand how subscripts and coefficients work when using chemical formulas. However the inclusion of one or more balancing coefficients is typically necessary for most chemical equations.
A coefficient which is written directly before the chemical formula with which it is associated is defined as a whole-number value that indicates how many of the corresponding atom or molecule are involved in the overall chemical reaction. If you want to write it as a reminder and placeholder that is totally fine. Is CH4 2O2 CO2 2H2O a balanced equation.
The numerical coefficients in chemical equations show the ratio of the molecules of each substance involved in a chemical reaction both reactants and products. Some of the worksheets for this concept are Understanding chemical formulas Balancing chemical equations work Word equations and balancing equations Naming compounds chemical reactions and balancing Chemical equation practice identifying subscripts Name atoms are not or. Coefficients appear on the left side of a chemical formula.
It shows how many atoms or molecules of the substance are involved in the reaction. For example two molecules of hydrogen would be written as 2H 2. Each individual substances chemical formula is separated from others by a plus sign.
Balancing Chemical Equations with Interfering Coefficients Vocabulary Coefficient. Use coefficients to balance equations CH4 2O2 CO2 2H2O C1 H4 O4 C1 H 4 O4 Equation is now balanced. In order to balance this equation coefficients should be written in the blanks as necessary.
It is not wrong per se but it goes against conventions. It shows how many atoms or molecules of the substance are involved in the reaction. Coefficients are the numbers in front of the formulas.













