First Class Balancing Equations Examples

Therefore in a chemical reaction.
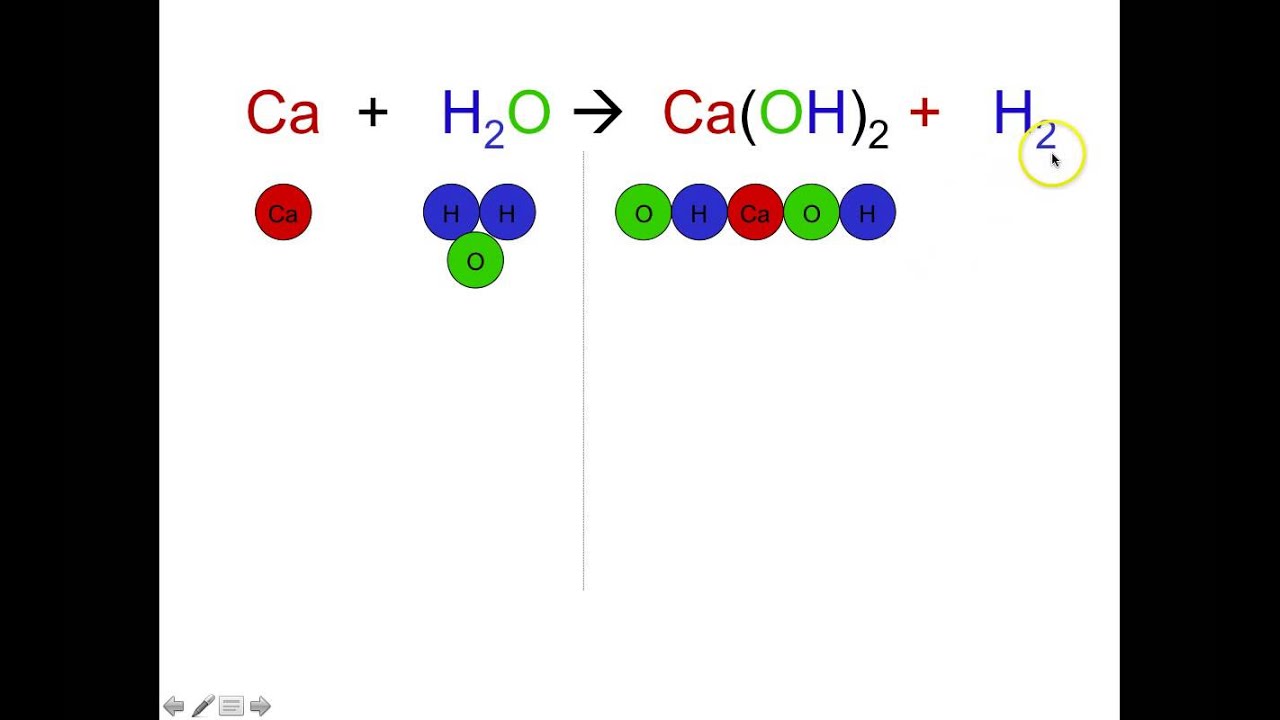
Balancing equations examples. One of the most engaging games comes from PHET. For example the total number of oxygen atoms in the reacting species 2O 2 is 4. To balance the Oxygen atoms add the appropriate number of water H2O molecules to the other side.
Check to make sure that all coefficients are in the lowest-possible ratio. C 6 H 12 O 6 O 2 CO 2 H 2 O. For instance 2H2 O2 - 2H20 denotes that there are four atoms of hydrogen and 2 atoms of oxygen on both sides of the equation.
The values of all the algebraic variables are substituted into the chemical equation to obtain the balanced chemical equation. This is a set of worked examples of how to balance chemical equations a very important skill in chemistry. When balancing equations remember chemical reactions must satisfy conservation of mass.
They range in difficulty level so dont get discouraged if some of them seem too hard. But this is completely contrary to what games can do at keeping you engaged. Worked Example Problem Tin oxide is heated with hydrogen gas to form tin metal and water vapor.
Both of them are separated by an arrow. The Balancing Equations Game from PHET Now an application can only go so far as to keep you engaged. A coefficient number in front of a chemical is multiplied by all the atoms in that chemical.
Example of balancing chemical equation by the algebraic method. 12or 52or 132 for example. The equation is balanced by adjusting coefficients and adding H2O H and e- in this order.