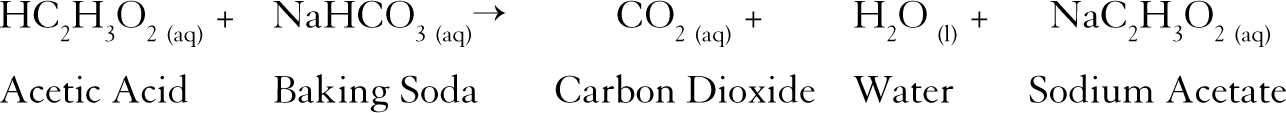Outrageous Baking Soda And Vinegar Reaction Type

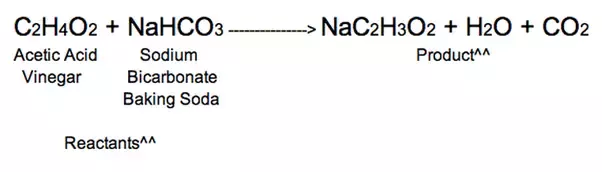
Reaction Between Baking Soda and Vinegar The first reaction is a double displacement reaction while the second reaction is a decomposition reaction.
Baking soda and vinegar reaction type. The reaction proceeds in two steps. The result of this initial reaction is two new chemicals. He also built a volcano to react the vinegar and baking soda in.
Adding vinegar to baking soda gives you an immediate reaction. Pour the baking soda into the balloon using a funnel. Repeat for each type of vinegar.
Baking soda and vinegar react to neutralise each other vinegar is an acid and baking soda an alkali releasing carbon dioxide which is the bubbles of gas you see. The balloon will be flopped to one side Lift the balloon up and pour the baking soda into the bottle of vinegar. Which vinegar made the reaction last longer with baking soda.
The three types of vinegar used were white vinegar red wine vinegar and balsamic vinegar. The baking soda and vinegar reaction is actually two separate reactions. The more vinegar you add the longer the bubbles will expand.
Vinegar isnt just an acid it is an acid in water which is important. More vinegar is better. Baking soda is a base and vinegar is an acid.
Carbonic acid and sodium acetate. The second reaction is a decomposition reaction. When vinegar and baking soda are first mixed together hydrogen ions in the vinegar react with the sodium and bicarbonate ions in the baking soda.