Beautiful Work Write A Balanced Equation For The Complete Combustion Of Propane

If 180 g of CO2 were produced from the complete combustion of propane how many grams of C3Hs were consumed in the reaction.
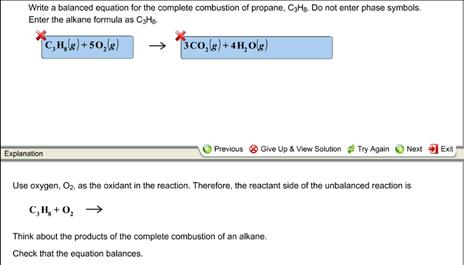
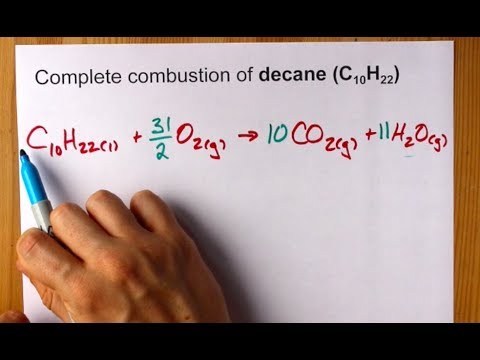
Write a balanced equation for the complete combustion of propane. By signing up youll get. Chemistry questions and answers. Write and balance equation for the complete combustion of decane C10H22.
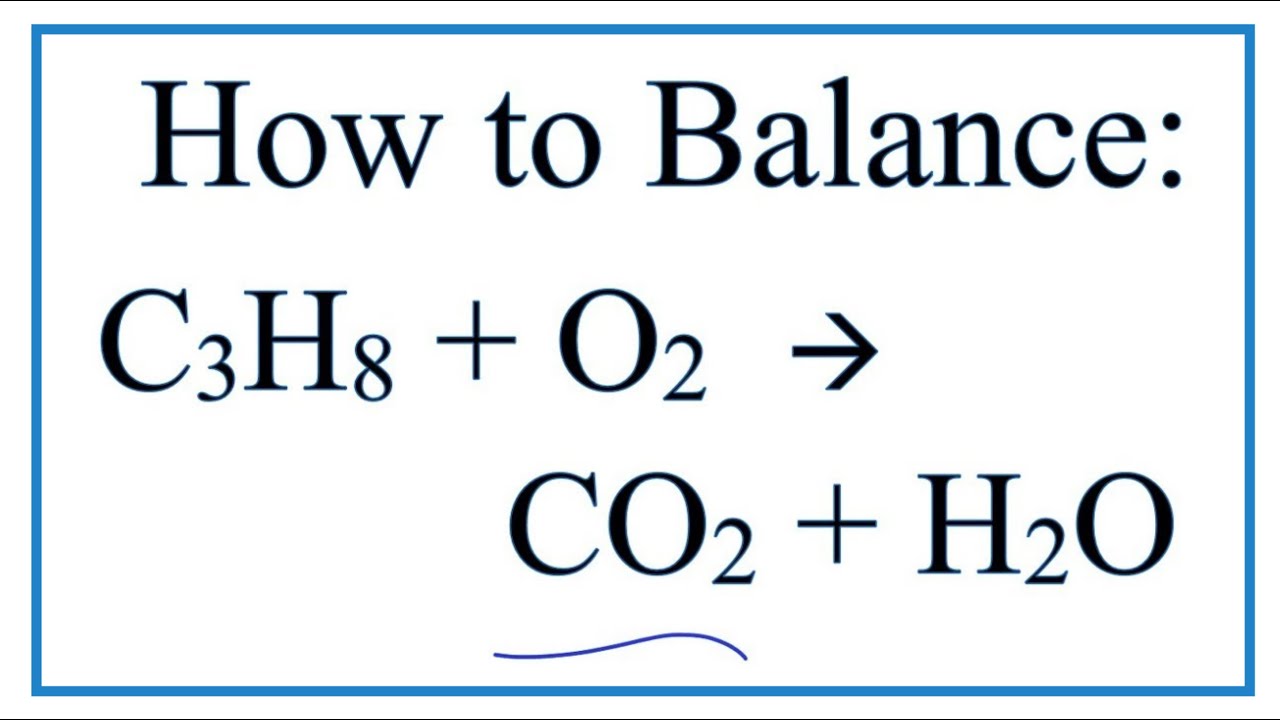
The formula for propane is C3H 8. Phases Are Optional Equation. Assume that air is 210 percent O2 by volume.
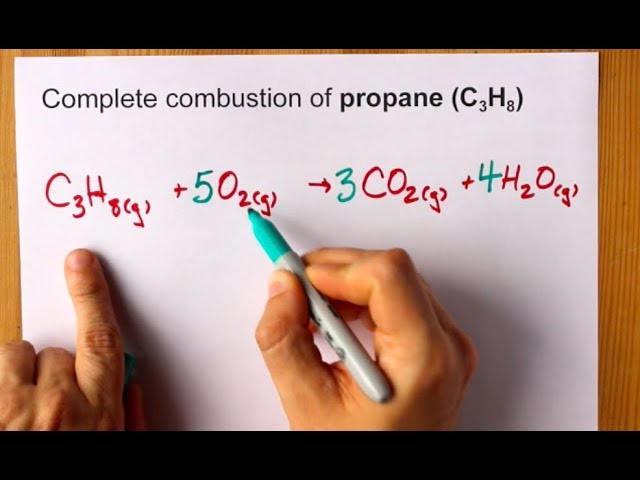
Write a balanced equation for the complete combustion of propane gas which yields CO2g and H2Ol. Write A Balanced Equation For The Complete Combust. Calculate the volume of air at 30C and 100 atmosphere that is needed to burn completely 100 grams of propane.
Write out a balanced equation for the combustion of the hydrocarbon compound propane C3H8 reacting with 02. Propane C 3 H 8 is a hydrocarbon that is commonly used as fuel. 2 C4H10 13 O2 -- 8 CO2 10 H2O.
Write a balanced chemical equation for the combustion of gaseous propane in gaseous oxygen to produce gaseous carbon dioxide and liquid water. Assume that air is 210 percent O 2 by volume. Write a balanced chemical equation for the combustion of propane c3h8 if you begin with 100l of propane how many liters of oxygen are required for complete combustion write the balanced chemical reaction for the complete combustion of propane c3h8 you can use shorthand notation a make sure to include all reactants and all products b.
Write A Balanced Equation For The Complete Combustion Of Propane CH. BBC Bitesize - GCSE Chemistry - Alkanes And Alkenes. C3H85O23CO24H2OThe combustion of pentane C5H12 follows this reaction.