Outstanding What Is The Difference Between A Skeleton And Balanced Chemical Equation

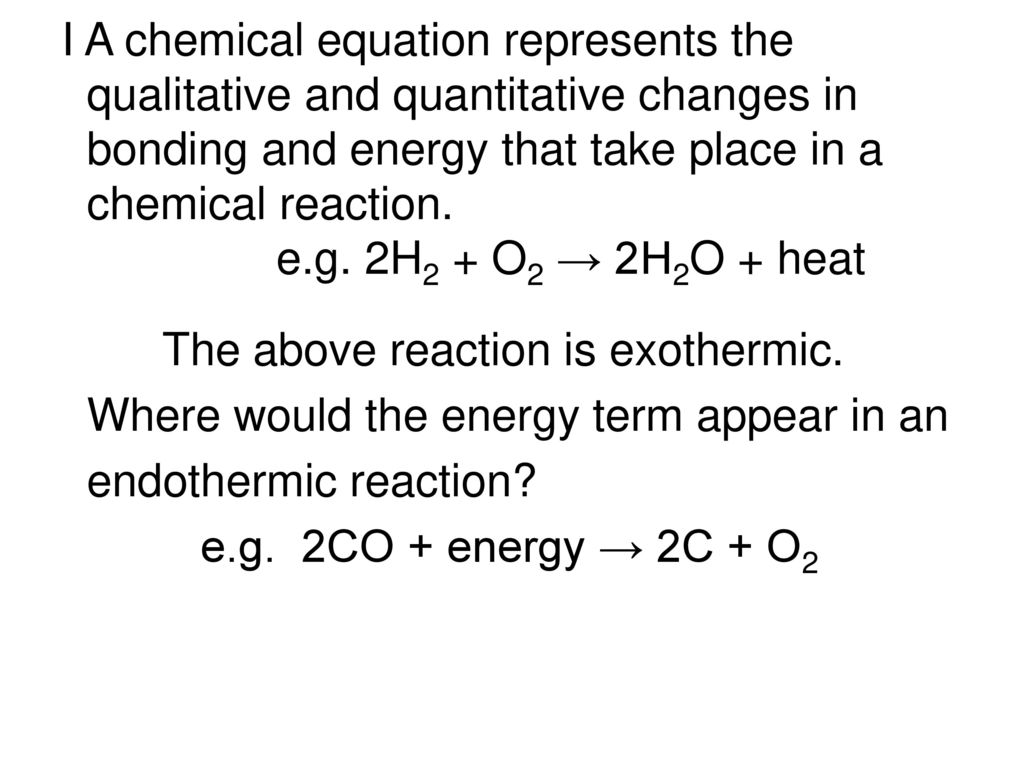
What do equations not do during a reaction.
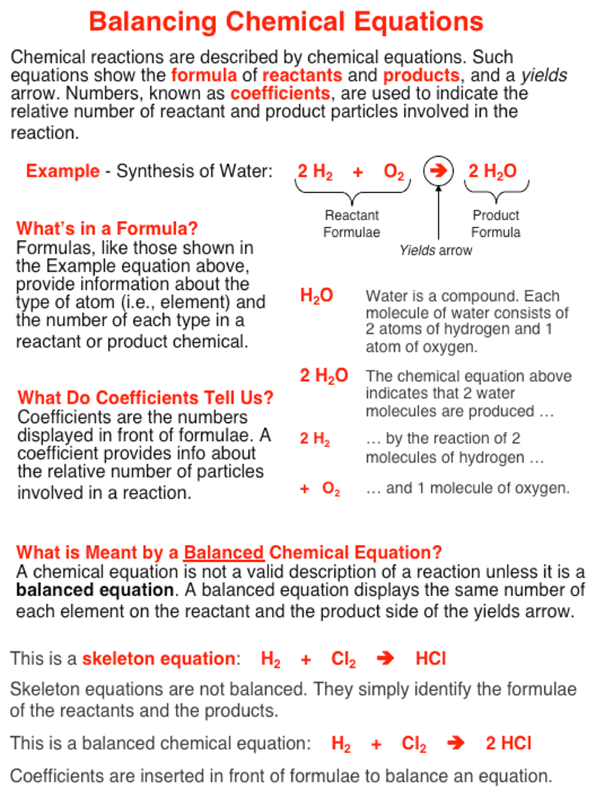
What is the difference between a skeleton and balanced chemical equation. Differentiate between a skeletal chemical equation and a balanced chemical equation. Later it has to be balanced. This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide.
Class X Chemical Reactions Equations In Hindi Class 10 Youtube. Skeleton equations are more informative. Differentiate Between a Balanced and a Skeletal Chemical Equation.
What is a balanced chemical equation. The chemical equation in which number of atoms are not equal on both sides is called a skeletal chemical equation. Asked Apr 12 2017 in Chemistry by Annu Priya 212k points chemical reactions and equations.
Skeleton reaction or unbalanced equations are very important in chemistry. It describes a chemical reaction using the chemical formulas of the reactants and products. Mg HCl MgCl 2 H 2.
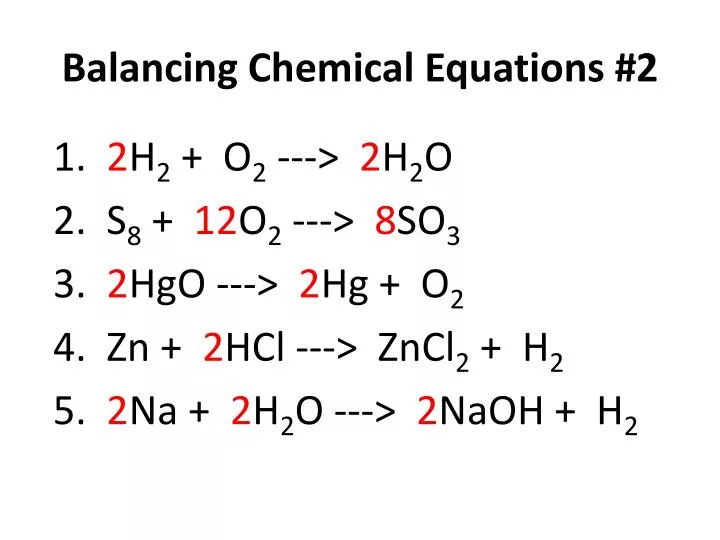
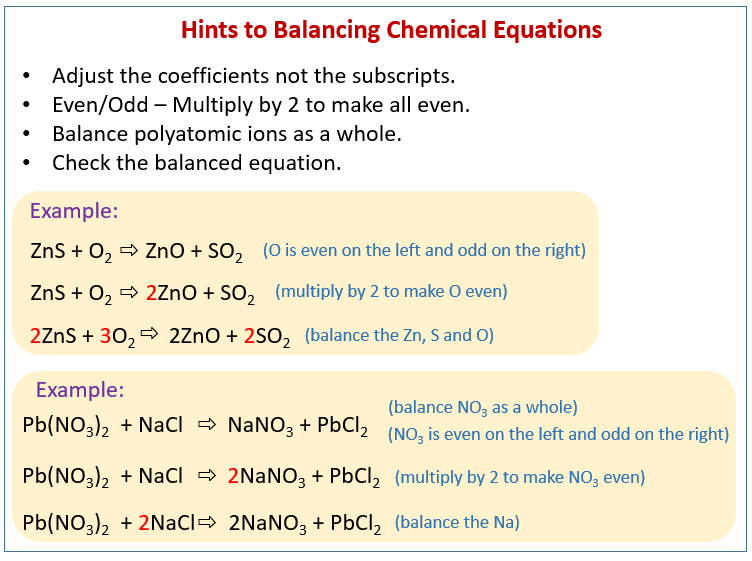
In balanced chemical equation the number of atoms of each element on both the sides of the equation are equal. How To Balance A Chemical Equation Class 10 Science Youtube. A balanced chemical equation is one in which the number of atoms each element on the reactant side is equal to the number of atoms of that element on the product side.
Write the skeleton equation. 1Mg 2O 1Mg 1O 3. In a balanced chemical reaction the number of atoms in each element of a reactant side should be equal to the number of atoms of that element on the product side.