Breathtaking Test Tube With Least Rusted Iron Nails

Calcium chloride in the right-hand test tube absorbs water The nail only rusts in the left-hand test tube.
Test tube with least rusted iron nails. A student set up six test tubes to investigate the rusting of iron. In which test tubes the rusting of iron nail will take place. A b and d.
Oil being more dense keeps floating on water and thus the iron nail does not come in contact with oil. Since rust is reddish brown in colour the nails will turn reddish brown in colour as well. The iron goes into solution as ferrous acetate and bubbles of hydrogen gas are released to the atmosphere.
Write the formula of rust. Rusting of iron takes place only in presence of oxygen and water vapours. 9 Almost fully rusted all over the entire nail with minimal signs of the original metal.
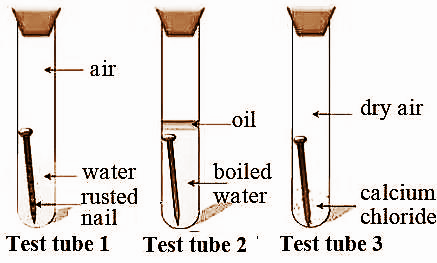
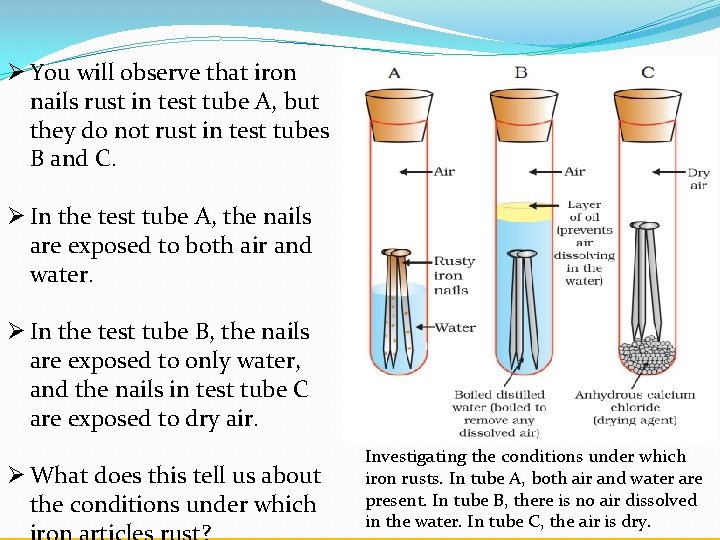
Almost all metals except gold and a couple of other precious metals will corrode in this fashion combining with oxygen and forming an oxide of the metal in question. Iron nails rust in test tube A but they do not rust in test tubes B and C. Put an iron nail in a dry test tube and label it C and plug with a small piece of cotton wool on which a few pieces of calcium chloride are placed.
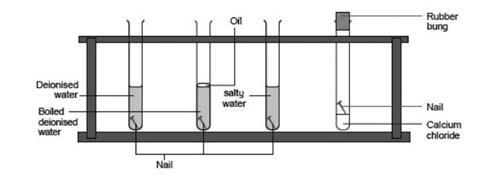
Set up the 6 test tubes or cups as shown in the picture above. Remove rust form any kind of metal or steel with this home made trick fast cheap and easy solution. In testtube B in testtube C b Before putting the iron nail in testtube D Jessica weighed the nail.
Plain water contains dissolved oxygen that will combine with the iron in the nail to form iron oxide also called rust and a very small amount of heat. Arrange the following test tubes from the nail that will rust most to the nail that will rust the least. A b and d.