Stunning Propane Combustion Chemical Equation

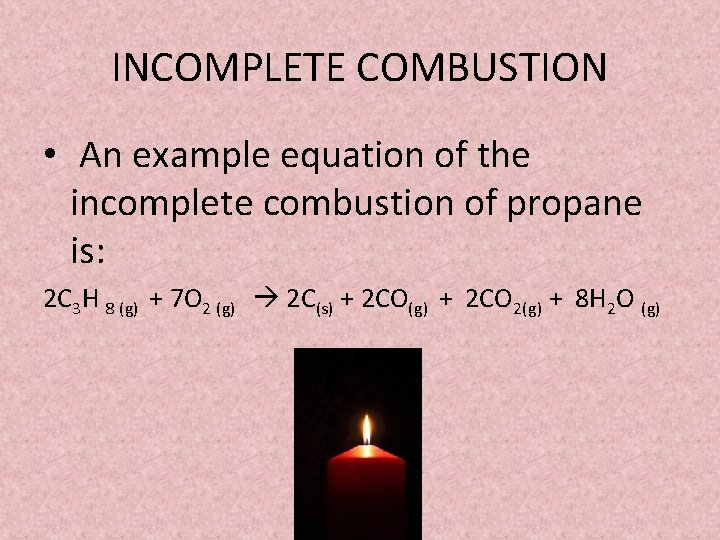
2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat.
Propane combustion chemical equation. The result of incomplete combustion is once again water vapour carbon dioxide and heat. The oxidation of a hydrocarbon such as propane is a combustion. Propane has a chemical formula of C3H 8 Combustion is when a hydro-carbon like propane containing carbon and hydrogen is burned in oxygen releasing carbon dioxide and water.
The balanced chemical equation for the combustion of propane is. C5H128O25CO26H2OThe combustion of octane C8H18 follows this reaction. The combustion of propane may be described by the chemical equation CH 50 300 4H09 How many grams of O g are needed to completely burn 21 g C H g.
The reaction can be summarized in the following chemical and word equations. Use heat contents of 915 x 106 Btu103 gallon for propane 102 x 106 Btu103 gallon for butane 1020 x 106 Btu106 scf for methane when calculating an equivalent heat input basis. The equation for incomplete combustion of propane is.
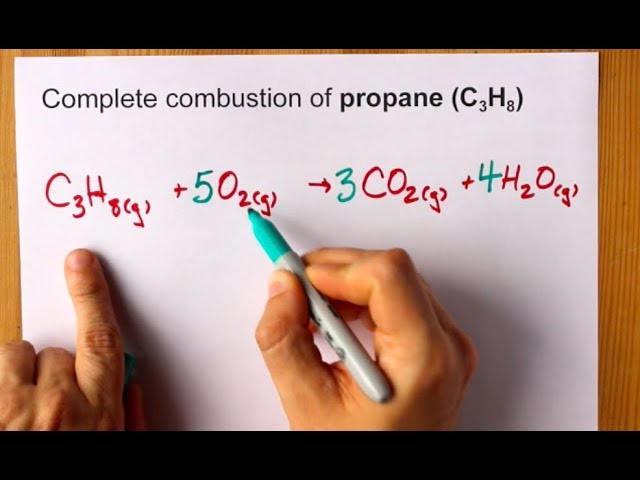
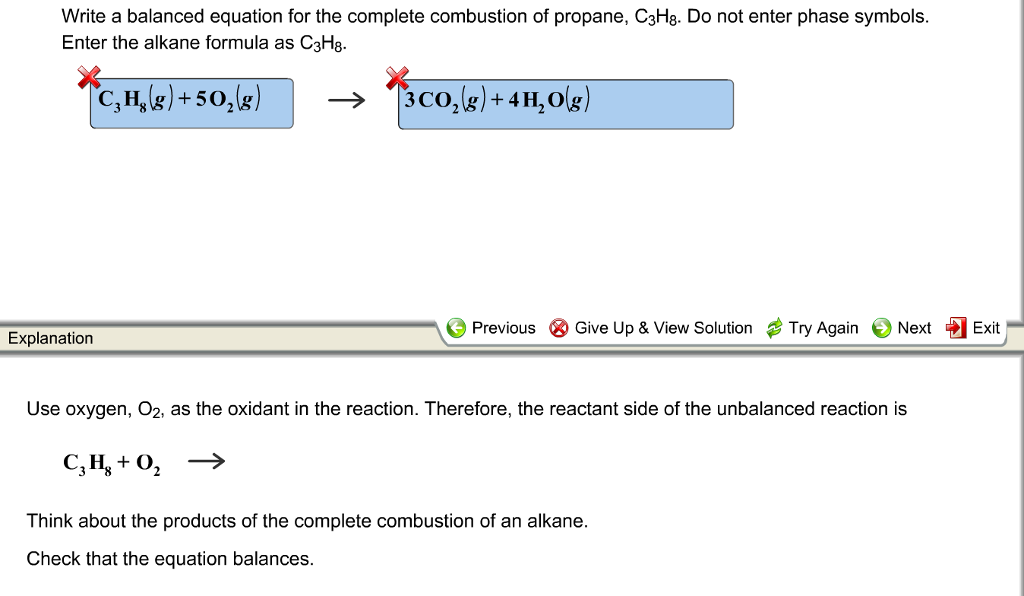
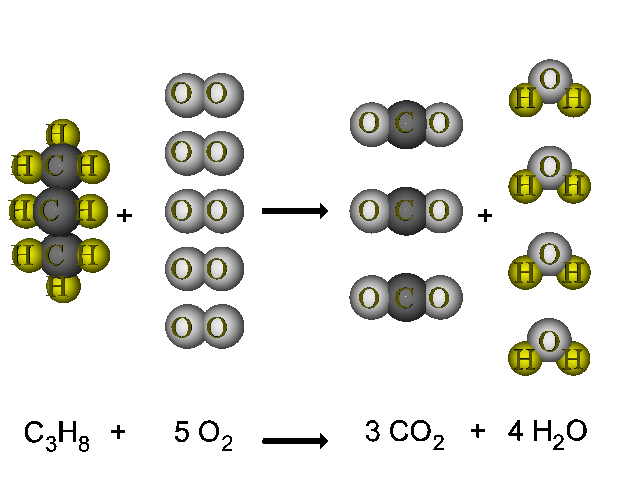
But it also produces carbon monoxide. C 3 H 8 g 5 O 2 g 3 C O 2 g 4 H 2 O g. In the simplest combustion process known as Stoichiometric Combustion.
Find step-by-step Biology solutions and your answer to the following textbook question. For example the equation for converting from methanes emissions factors to propanes emissions. With a word formula itll be.
C3H85O23CO24H2OThe combustion of pentane C5H12 follows this reaction. Propane plus oxygen becomes carbon dioxide plus water. The basic equation looks like thisThe combustion of propane C3H8 follows this reaction.