Unbelievable Octane Burns In Oxygen Balanced Equation

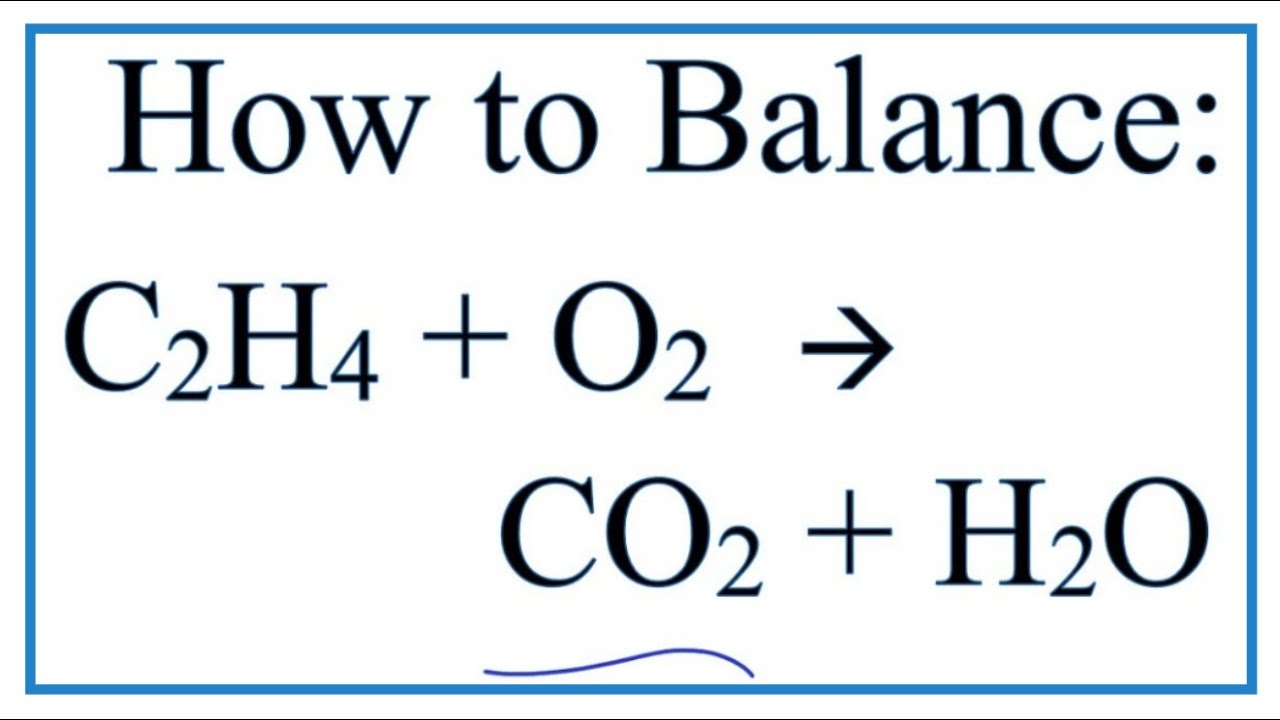
Write an unbalanced formula equation for the reaction.
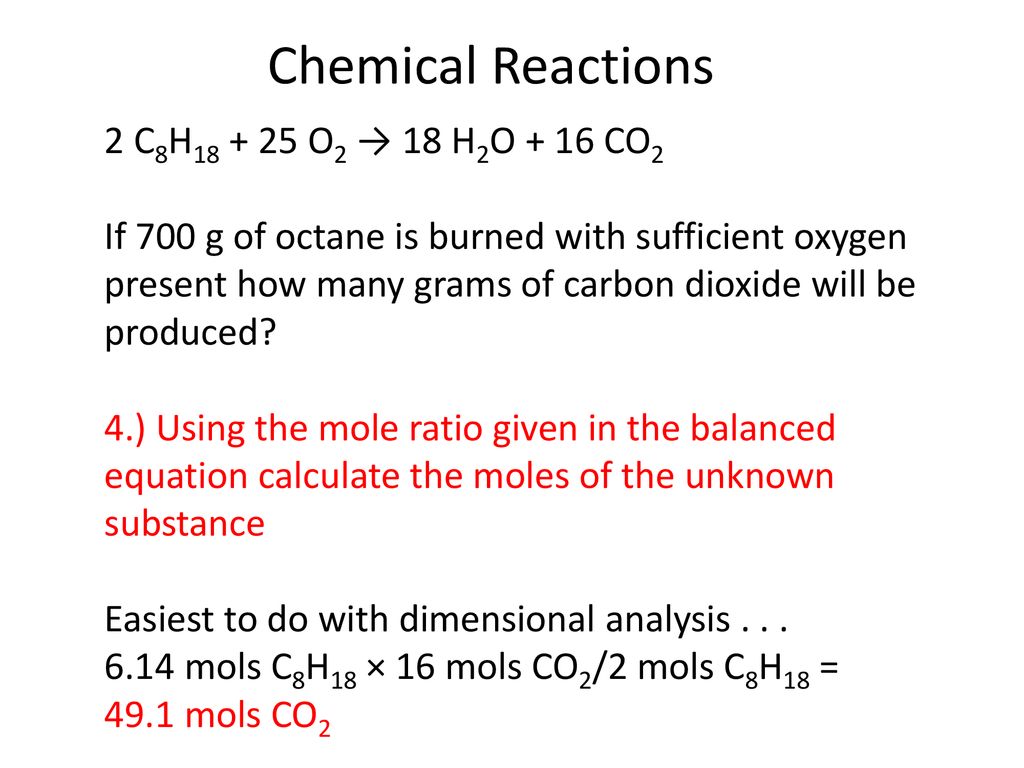
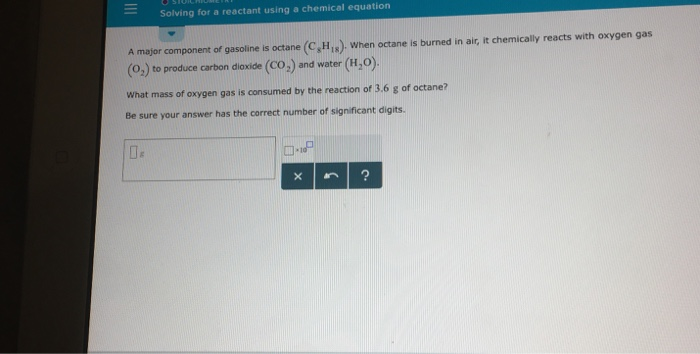
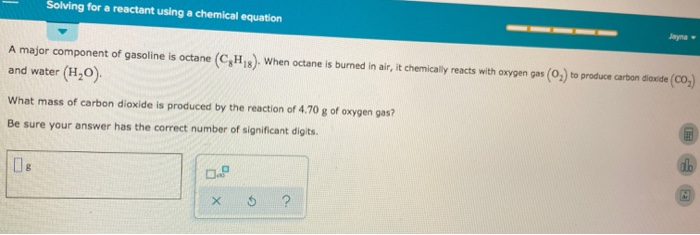
Octane burns in oxygen balanced equation. Octane Oxygen ---- Carbon dioxide Water. 2CH2 250 16CO 18H2O i How many moles of carbon dioxide are produced by one mole of octane. Write the balanced molecular equation for the complete combustion of octane.
The little number written at the lower right after an atom subscript tells how many of that atom are in the molecule. So Much More Address. 914 963 - 0183.
The balance equation for this reaction is C3H8 5O2 ---. Write a balanced chemical equation for this process. Indicate the states of the reactants and products.
Write a balanced chemical equation for this process. Calculate the volume of carbon dioxide produced at 25C and a pressure of 101105 Pa when 985 g of octane is used up in the combustion reaction. Complete Combustion of Octane C8H18 Balanced Equation â 028 moles of octane will produce 0289 252 moles of water.
The combustion of a hydrocarbon produces carbon dioxide and water. Calculate the volume of carbon dioxide produced at 25 degrees Celsius and a pressure of 101105 101 10 5. Ii How many moles of oxygen are required to react completely with one mole of octane.
What is the amount in moles of water produced from 051 mol C2H5OH. Use the smallest whole number coefficients. The balanced chemical reaction is 2C8H 18 25O2 16CO2 18H 2O Heres a nice diagram of this reaction Ive found on a French forum.