Exemplary Incomplete Combustion Butane

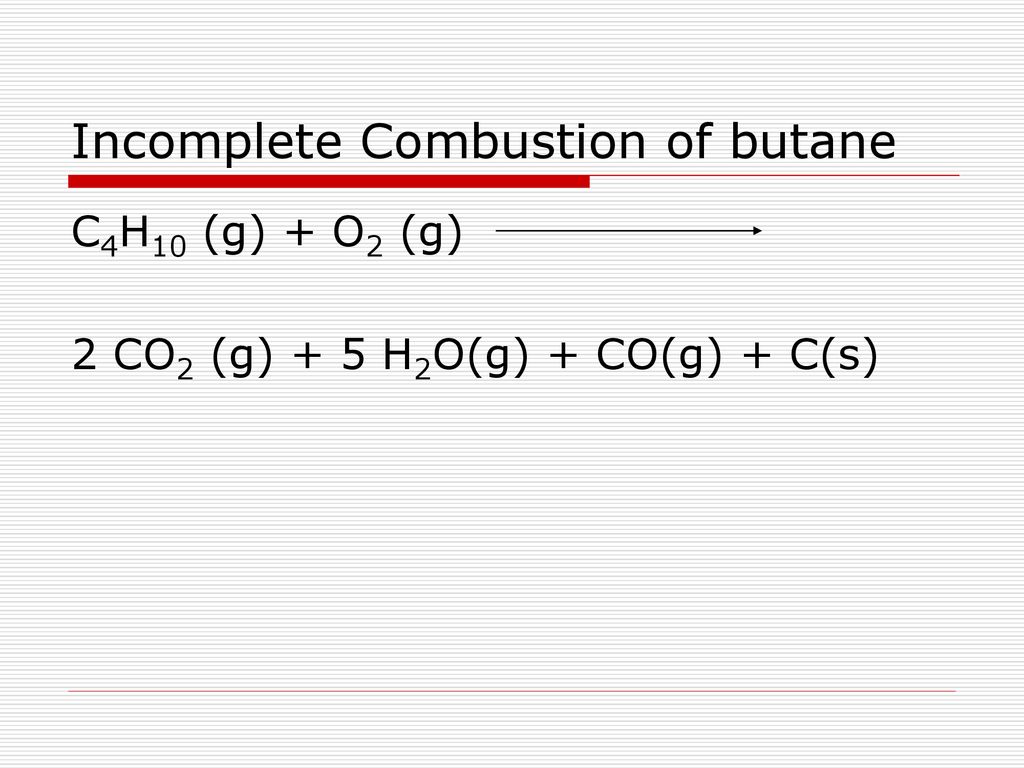
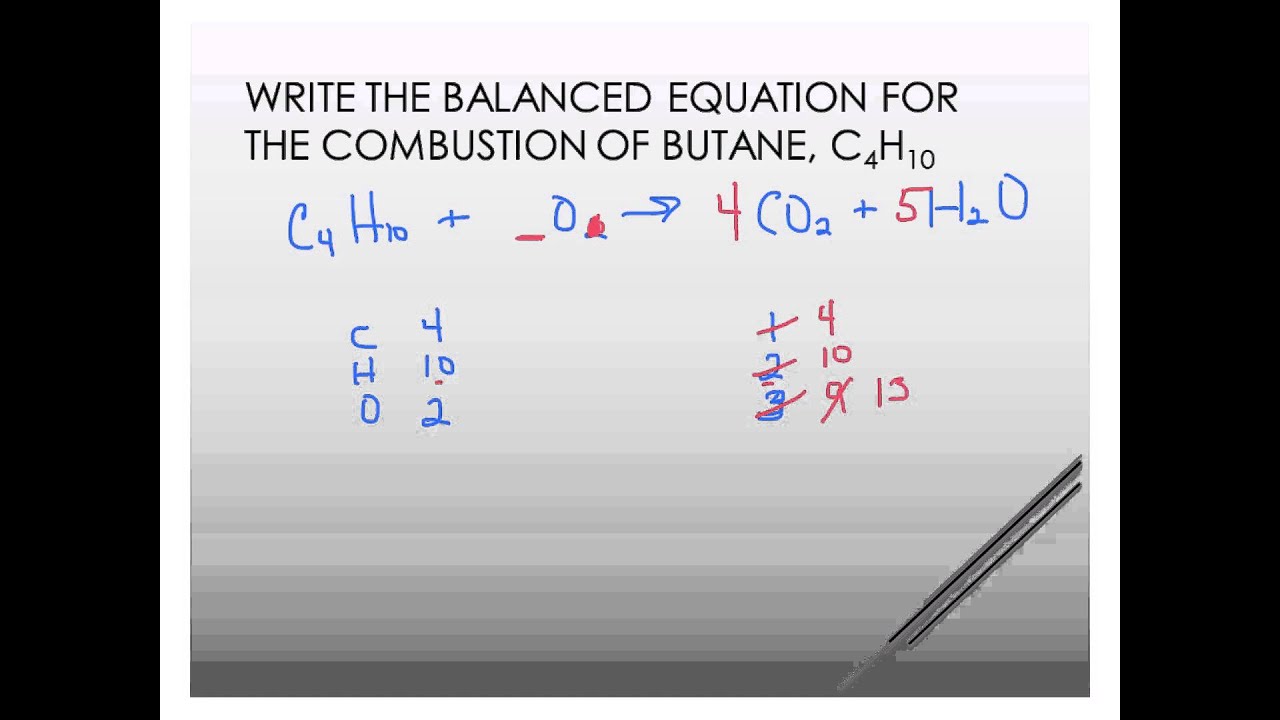
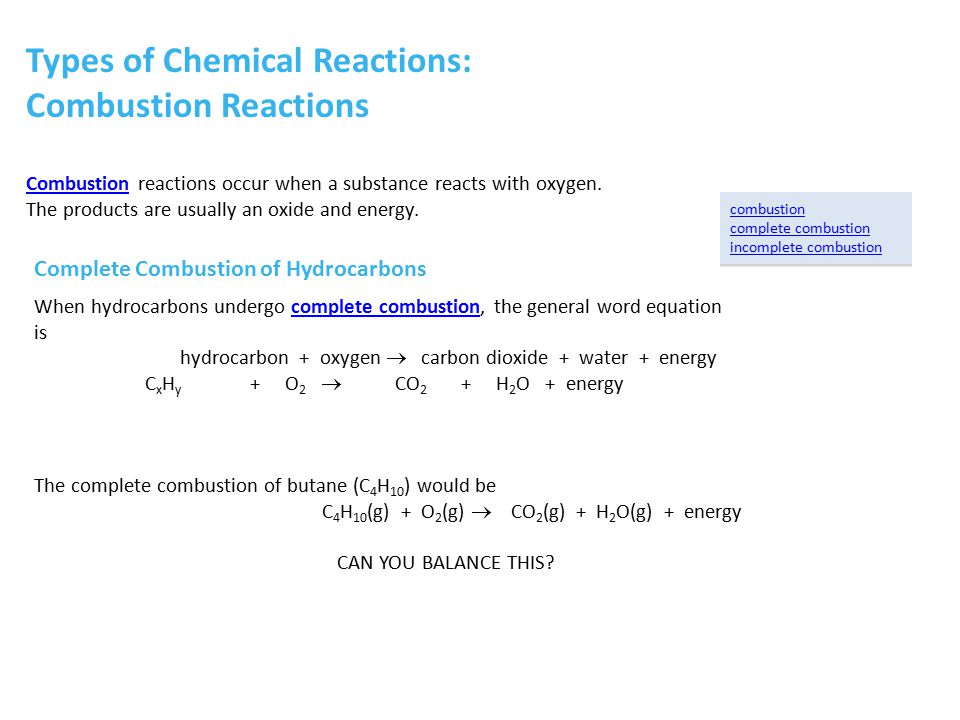
Complete combustion of butane will produce carbon dioxide and water but incomplete combustion not enough oxygen will produce carbon monoxide.
Incomplete combustion butane. What are the differences. H_2S on incomplete combustion with oxygen forms mainly. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide.
Incomplete combustion is defined as the type of reaction in which a hydrocarbon reacts with oxygen to produce carbon monoxide water and carbon. What is the heat liberated by buring 1. Could it happen the reaction should be described by the equation C2H6 2 O2 CO C 3 H2O From a purely stoichiometric standpoint it is to be pointed out that in the absence of the arbitrary constraint concerning the equal amounts of C.
I need to write an equation for the incomplete combustion of butane to form a mixture of carbon monoxide unburnt carbon and water. Butane is undergoing incomplete combustion as shown by the yellow flame. Incomplete combustion where there is not enough oxygen present can lead to the formation of carbon or carbon monoxide.
The carbon is released as soot. It has a role as a food propellant and a refrigerant. One mole of butane requires 65.
La combustion incomplète du butane video sciences physiques physique chimie collège 4ème quatrième Nicolas Braneyre CLG Zola. General equation for incomplete combustion reaction follows. 01 mole of butane will use 01 45 045 moles.
The heat of combustion of butane is 2880kJ mol-1. Vanklik Mon 12212009 - 0046. For incomplete combustion of butane ie.