Matchless Hsc Periodic Table

2013 HSC Chemistry Marking Guidelines Section I Part A Multiple-choice Answer Key Question Answer 1 B 2 C 3 D 4 C 5 A 6 A 7 C 8 A 9 A 10 C 11 B 12 B 13 D 14 B 15 C.
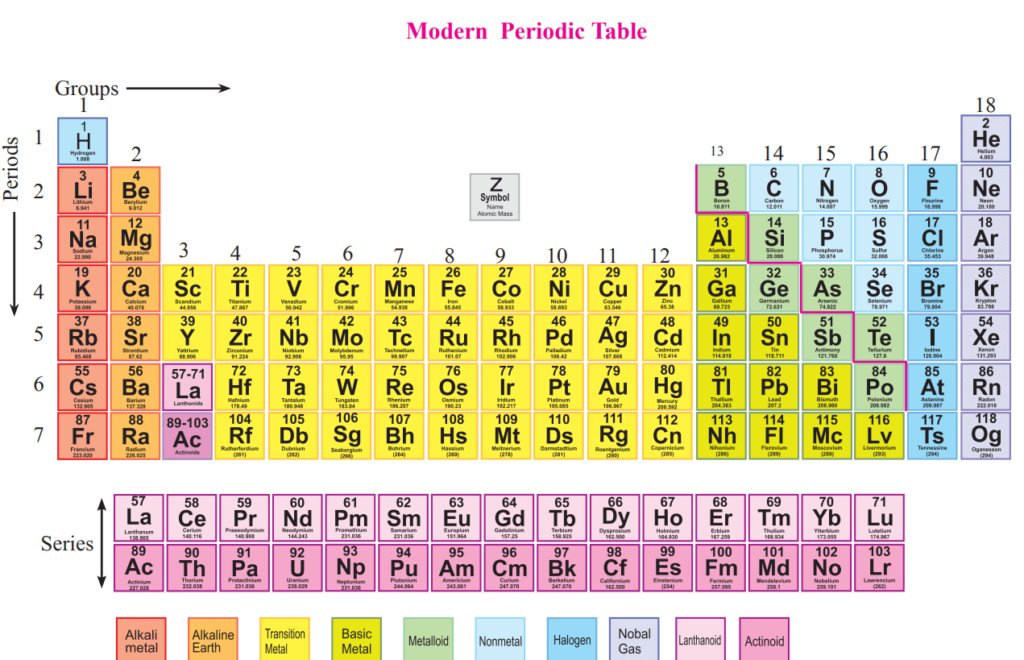
Hsc periodic table. Describe the background of development of periodic table the relation of the electronic configuration of outermost energy level ofthe elements to the princi. Click the tabs at the top to explore each section. Just below the answer you will be guided to the complete puzzle.
Due to this reason the lanthanoids are placed at the bottom of the periodic table with a reference to the third group in the sixth period. All of these elements display several other trends and we can use the periodic law and table formation to predict their chemical physical and atomic properties. The d-block elements are present in 4 th period Sc to Zn 10 elements 5 th period Y to Cd 10 elements 6 th period La Hf to Hg 10 elements and 7 th period Ac Rf to Uub 10 elements.
You are in the right place and time to meet your ambition. NSW Education Standards Authority Created Date. 1 2019 higher school certificate examination chemistry.
The d-block elements lie in between s- and p-block elements ie these elements are located in the middle part of the periodic table. It provides a comprehensive summary of the three core topics. 2 3.
Referral to periodic table and chlorine being more electronegative than bromine 11 2013 HSC Chemistry Marking Guidelines. This position in the periodic table is justified due to following facts. This will clear students doubts about any question and improve application skills while preparing for board exams.
Hsc Chemistry free download - ChemLab Periodic Table And Chemical Solutions Calculator IQ Flash Cards and many more programs. The number of valency electrons is same for all elements ie one in 5d and two in 6s. Name the elements - see how many chemical elements you can name in 10 minutes.