Wonderful Endothermic Reaction Formula

7311 CaCO 3 s CaO s CO 2 g Δ H 1778 kJ.
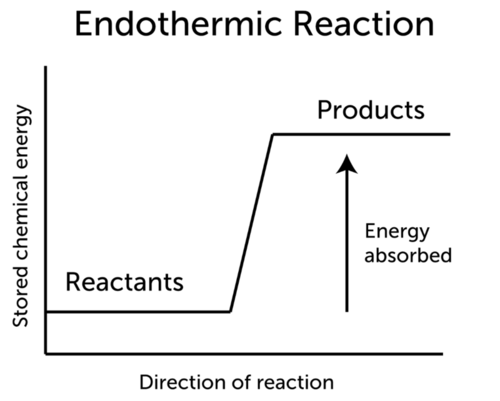
Endothermic reaction formula. The energy change that accompanies a reaction can be written in the chemical equation. Reactants Products Energy. The chemical equation can be written as follows.
Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Besides using the term heat in the equation we can also show the nature of the reaction by enthalpy change. All combustion reactions are exothermic reactions.
This video is very helpful for 10th board students. For this it helps to know a bit about chemical reactions and chemical bonds. When 1 mol of calcium carbonate decomposes into 1 mol of calcium oxide and 1 mol of carbon dioxide 1778 kJ of heat is absorbed.
An endothermic reaction uses energy as a reactant. One of the most important series of endothermic reactions is photosynthesis. Energy in the form of sunlight is absorbed during the reaction.
In a chemical equation the word heat or the triange symbol are used to indicate if the reaction is exothermic or endothermic. As a result the products are likely to be warmer than the reactants. An endothermic process or reaction absorbs energy in the form of heat endergonic processes or reactions absorb energy not necessarily as heat.
The general equation for an exothermic reaction is. A few examples of the endothermic process are photosynthesis evaporating liquids melting ice dry ice alkanes cracking thermal decomposition ammonium chloride in water and much more. The sign for the energy change is.